��Ŀ����
��δ�ʯ���л�ø��������ȼ��һֱ�ǻ�ѧ��̽���Ŀ��⣬��ʯ�ͷ���õ������ͽ����ѻ����Ի�ø��������ȼ�͡�
����һ��ʯ���Ǻ���20��30��̼ԭ�ӵ������Ļ��������³ʹ�̬��
���϶���ʯ�ʹ��ѻ���ͨ��ʹ��Al2O3��������
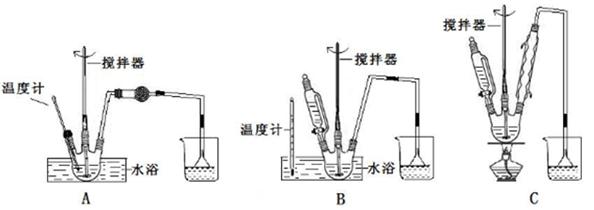
ij�о���ѧϰС����ʵ������ģ��ʯ�͵Ĵ��ѻ���װ����ͼ��ʵ������пɹ۲쵽��ƿ�й���ʯ�����ۻ����Թܢ���������Һ�����ᣬ�Թܢ������Ը��������Һ��ɫ��ʵ������Թܢ���Һ����ζ���������͵���ζ��
(1)��װ���������ӵ�˳��Ӧ��ѭ��ԭ��Ϊ__________________��
Ϊ��֤ʵ��ɹ���ʵ��ǰ������еIJ�����_________________��
װ���нϳ����ܵ�������_____________________��
(2)�Թܢ�������Һ������˵����______��
(3)�Թܢ�����Һ��ɫ˵����________________��
(4)�ܷ����Թܢ��е�Һ����ȡ��ˮ�е��壬������_______________��
(5)д����ʮ���ѻ��õ������ϩ�Ļ�ѧ����ʽ________________��
(6)ʯ���ѻ�����Ҫ������_____________��
(1)�������ϣ��������ҡ����װ�õ������ԡ���������������
(2)�ѻ�������̼ԭ��������4����
(3)�ѻ�������̼ԭ����С��5��ϩ��
(4)���ܣ���Ϊ�ѻ���������ϩ���������巢���ӳɷ�Ӧ
(5)C20H42 C10H22��C10H20
C10H22��C10H20
(6)�����ʯ�Ͳ�Ʒ������ȼ�ͣ��ر������͵IJ���������
����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����ʵ���ܴﵽԤ��Ŀ�ĵ���
| ��� | ʵ������ | ʵ��Ŀ�� |
| A | ��CO2����HC1���ʣ�ͨ�뱥��NaHCO3��Һ�� | ��ȥHC1 |
| B | ������������Ӧ����Թ��м���ϡ��ˮ | ��ȥ�Թ��ڲ����� |
| C | ���������ˮ������Һ��ֱ�Ӽ������Ƶ�Cu(OH)2�������� | ��������Ƿ�ˮ�� |
| D | ������FeC12������ˮ�ܽ⣬��ϡ�����ữ���ٵμ�KSCN��Һ | ����FeCl2�Ƿ���� |
����ʵ����ȷ����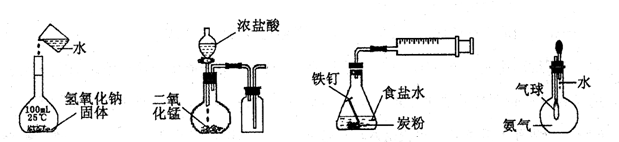
| A������һ��Ũ����Һ | B����ȡ���ռ�Cl2 | C���������ⸯʴ | D����֤����������ˮ |
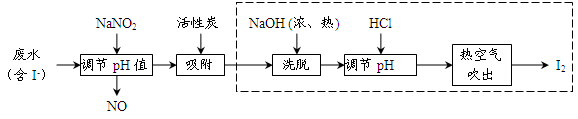
��14�֣�����34Se�����ڣ�52Te�����ǵ�VIA��Ԫ�أ����Ƿֲ��ڵؿ��е�ϡ��Ԫ�ء���ҵ�����ķ��ϣ���Ҫ�ɷ������ڡ�̼��ͭ�����Ͻ𣩻��վ��������������£�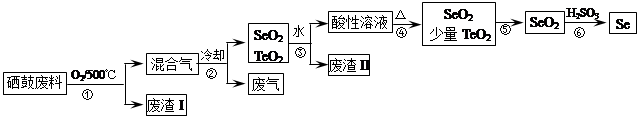
��֪��
| �������� | �۵� | �е� | ���� | �ܽ��� |
| SeO2 | 340�� | 684�� | 315�� | ������ˮ |
| TeO2 | 733�� | 1260�� | 450�� | ����ˮ |
��2���������ͨ�������ʹ���ķ��Ϸ��ڣ�Ŀ����______��
��3����������Ҫ�ɷ���______������II����Ҫ�ɷ���______��
��4�����������Ҫ��Ӧ�Ļ�ѧ����ʽ��______�������Ӧ�Ļ�ѧ����ʽ��______��
��5�����ݱ������ݣ�����������˵ķ��뷽����______��
�����������һ�ֿɴٽ�����������Ӫ�����ʡ���������ƿ�ͨ�����·�Ӧ�Ƶã�
C6H12O6(������)��Br2��H2O��C6H12O7(��������)��2HBr
2C6H12O7(��������)��CaCO3��Ca(C6H11O7)2(���������)��H2O��CO2
������ʵ��ܽ��Լ��±���
| �������� | ��������� | �������� | �廯�� | �Ȼ��� |
| ˮ�е��ܽ��� | ��������ˮ ��������ˮ | ���� | ���� | ���� |
| �Ҵ��е��ܽ��� | �� | �� | ���� | ���� |

��ش��������⣺
��1���ڢٲ�����ˮ����������ʱ������װ������ʵ���________��
�Ʊ���������ƵĹ����У������ǵ�����Ҳ���������Լ����������������ʺϵ���________��
A������Cu(OH)2����Һ B������KMnO4��Һ
C��O2������������ø D��[Ag(NH3)2]OH��Һ
��2���ڢڲ���ַ�Ӧ��CaCO3��������ʣ�࣬��Ŀ����________����ʵ���в�����CaCl2���CaCO3��������________��
��3���ڢ۲�����ȹ��ˣ���ԭ����________��
��4���ڢܲ������Ҵ���������________��
��5���ڢ��У�����ϴ�Ӽ�����ʵ���________��
A����ˮ B����ˮ C���Ҵ� D���Ҵ���ˮ�����Һ
�����й�ʵ��װ�ý��е���Ӧʵ�飬�ܴﵽʵ��Ŀ�ĵ��ǣ�������
| A������ͼ��a����ʾװ�ý���ϡ������ͭ�ķ�Ӧ��ȡ���ռ�NO |
| B����ͼ��b����ʾװ�ý�������֪Ũ�ȵ�����������Һ�ⶨ����Ũ�ȵ�ʵ�� |
| C����ͼ��c����ʾװ����ȡ����Cl2 |
| D����ͼ��d����ʾװ�ü�������ķ��� |
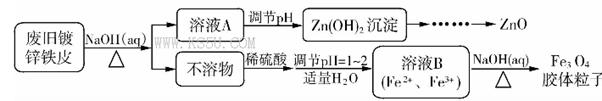
 Fe3O4�����壩+S4O62-+H2O
Fe3O4�����壩+S4O62-+H2O