��Ŀ����
ij�о�С���һԪ�л�����HA���ܼ�����ˮ�Ļ����ϵ�е��ܽ�̶Ƚ����о�����25��ʱ������HA��ˮ�в��ֵ��룬��HAŨ��Ϊ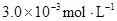 ʱ��������Ϊ0.20������ȣ��ѵ����HA������/��ʼHA���ܷ����������ڱ��в��ַ���˫�ۣ����ɣ�HA��2����ƽ����ϵ�У�һԪ�л�����HA���ܼ�����B����ˮ��W���еķ���ϵ��ΪK��K��C��HA��B��C��HA��W��1.0�����ﵽƽ����Է�����ʽ���ڵ�HA�ڱ���ˮ�����ܼ��еı���Ϊ1��1��������Ϣ���£�
ʱ��������Ϊ0.20������ȣ��ѵ����HA������/��ʼHA���ܷ����������ڱ��в��ַ���˫�ۣ����ɣ�HA��2����ƽ����ϵ�У�һԪ�л�����HA���ܼ�����B����ˮ��W���еķ���ϵ��ΪK��K��C��HA��B��C��HA��W��1.0�����ﵽƽ����Է�����ʽ���ڵ�HA�ڱ���ˮ�����ܼ��еı���Ϊ1��1��������Ϣ���£�
| 25��ƽ����ϵ | ƽ�ⳣ�� | �ʱ� | ��ʼ��Ũ�� |
��ˮ�У�HA  | 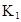 | 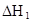 | 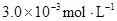 |
�ڱ��У�2HA 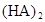 | 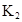 | 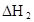 | 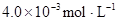 |
�ش��������⣺
��1������25��ʱˮ��Һ��HA�ĵ���ƽ�ⳣ��K1��___________��
��2��25�棬��ˮ��Һ��pHΪ___________������֪��1g2��0.3��lg3��0.5���ڱ���ϵ��HA��ת����Ϊ___________��
��3���ڱ��У�HA�������ۣ�2HA
 ��HA��2����Ӧ�ڽϵ��¶����Է����У���
��HA��2����Ӧ�ڽϵ��¶����Է����У���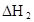 ___________0��
___________0����4��25������ϵ�У�HA�ڱ��з������ۣ������ijʱ����Һ����Ũ������
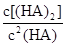 ��130����Ӧ��___________������С�
��130����Ӧ��___________������С�
��1�� mol/L ��2��3.2�� 40% ��3���� ��4����
mol/L ��2��3.2�� 40% ��3���� ��4����
���������������1��HA��ˮ��Һ�е��룺HA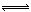 H++A-�����ݵ����Ϊ0.20��c��H+��=3.0��10-3mol/L��0.20=6.0��10-4mol?L?1����K=6.0��10-4mol?L?1��6.0��10-4mol?L?1�£�3.0��10-3mol?L?1��6.0��10-4mol?L?1��=1.5��10-4mol?L?1��
H++A-�����ݵ����Ϊ0.20��c��H+��=3.0��10-3mol/L��0.20=6.0��10-4mol?L?1����K=6.0��10-4mol?L?1��6.0��10-4mol?L?1�£�3.0��10-3mol?L?1��6.0��10-4mol?L?1��=1.5��10-4mol?L?1��
��2��pH=-lg��H+��=-lg��6.0��10-4��=3.2���ﵽƽ����Է�����ʽ���ڵ�HA�ڱ���ˮ�����ܼ��еı���Ϊ1��1��c��HA��=3.0��10-3mol?L?1��6.0��10-4mol?L?1=2.4��10-3mol?L?1��ת����HAΪ4.0��10-3mol?L?1��2.4��10-3mol?L?1=1.6��10-3mol?L?1�������ڱ���ϵ��HA��ת����Ϊ,1.6��10-3mol?L?��4.0��10-3mol?L?1��100% ="40%" ��
��3���÷�Ӧ�ڽϵ��¶����Է����У�T��Сʱ��?H-T?S<0����Ϊ��Ӧ���ʵ�����С����?S<0������?H<0
��4���ɣ�2�������ݿ����2HA ��HA��2��ƽ�ⳣ��K=8.0��10-4mol?L?1�£�2.4��10-3mol?L?1��2=146��
��HA��2��ƽ�ⳣ��K=8.0��10-4mol?L?1�£�2.4��10-3mol?L?1��2=146��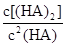 ��130<K�����Է�Ӧ������Ӧ������С�
��130<K�����Է�Ӧ������Ӧ������С�
���㣺���⿼��ƽ�ⳣ���ļ����Ӧ�á�pH��ת���ʵļ��㡢��Ӧ�Է����еķ�����

�ڳ��¡���ѹ�����������£�N2�ڴ���������ˮ�������з�Ӧ��
2N2 (g)+6H2O(l) 4NH3 (g)+3O2 (g) ��H�� a kJ��mol��1
4NH3 (g)+3O2 (g) ��H�� a kJ��mol��1
������ӦNH3���������¶ȵĹ�ϵ����ѹ�´ﵽƽ��ʱ��ò���ʵ���������±���
| �¶� T/K | 303 | 313 | 323 |
| NH3������/(10��6 mol) | 4.8 | 5.9 | 6.0 |
��2����ˮϡ��0.1 mol��L��1��ˮ����ϡ��ʱ��Һ�¶Ȳ��䣩������Һ������ˮ�������Ӷ���С�������е� ������ţ���
A��c(NH3��H2O) B��
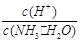 C��c(H+)��c(OH��) D��
C��c(H+)��c(OH��) D��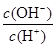
��3����ҵ�ð���ȡ�����������ӦΪ��4NH3(g)+5O2(g)
 4NO(g)+6H2O(g) ��H��0������ʼ�����ʵ�����ͬ�������й�ϵͼ��ȷ����________(�����)��
4NO(g)+6H2O(g) ��H��0������ʼ�����ʵ�����ͬ�������й�ϵͼ��ȷ����________(�����)��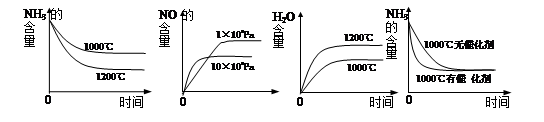
A B C D
��4����1L�ݻ��̶����ܱ������з���������Ӧ���������ʵ����ʵ���Ũ�����±���
| ʱ��/Ũ�� | c(NH3) (mol/L) | c(O2 ) (mol/L) | c(NO) (mol/L) |
| ��ʼ | 0.8000 | 1.600 | 0.000 |
| ��4 min | 0.3000 | 0.9750 | 0.5000 |
| ��6 min | 0.3000 | 0.9750 | 0.5000 |
| ��8 min | 0.7000 | 1.475 | 0.1000 |
��Ӧ�ڵ�6 min��8minʱ�ı����������ı������������___________________���ڸ������£�ƽ����_______�ƶ�(����ҡ�)��
��E��F����̶��ݻ����ܱ������У���һ�������·�����Ӧ��
E(g)+F(s) 2G(g)�����Թ��������ƽ��ʱG���������(%)���¶Ⱥ�ѹǿ�ı仯���±���ʾ��
2G(g)�����Թ��������ƽ��ʱG���������(%)���¶Ⱥ�ѹǿ�ı仯���±���ʾ��
| ѹǿ/MPa �������/% �¶�/�� | 1.0 | 2.0 | 3.0 |
| 810 | 54.0 | a | b |
| 915 | c | 75.0 | d |
| 1000 | e | f | 83.0 |
��2���÷�Ӧ�ġ�S 0���>������<����=������ͬ����b f��
��3��ƽ�ⳣ��K(1000��) K(810��) �������� ��
��4������������Ӧ������˵����ȷ���� ������ţ���
�ٻ��������ܶȲ��ٱ仯����Ӧ�ﵽƽ��
�ڸ÷�Ӧ������ӦΪ���ȷ�Ӧ
������F��������λ��������������ѧ��Ӧ���ʼӿ�
�ܺ��º���������ͨ��������壬ƽ�ⲻ�ƶ�
�ݺ��º�ѹ������ͨ��������壬��ѧ��Ӧ���ʼӿ�

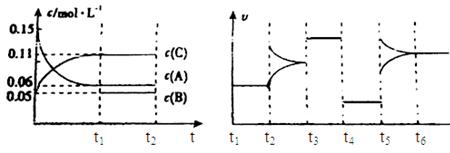
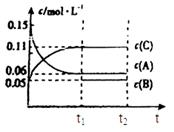
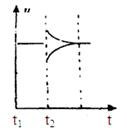
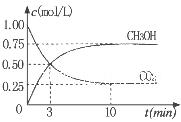
 CH3OH(g)���ݻ�Ϊ1L�����ܱ������зֱ����1molCO��2molH2��ʵ���ü״������ʵ������¶ȡ�ʱ��Ĺ�ϵ������ͼ��ʾ���������Ӧ�ġ�H_______0�����������������=�������жϵ�������______��
CH3OH(g)���ݻ�Ϊ1L�����ܱ������зֱ����1molCO��2molH2��ʵ���ü״������ʵ������¶ȡ�ʱ��Ĺ�ϵ������ͼ��ʾ���������Ӧ�ġ�H_______0�����������������=�������жϵ�������______��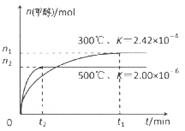
 CH3OH+H2O��
CH3OH+H2O��

 2SO3(g) ��H=��198kJ��mol-1
2SO3(g) ��H=��198kJ��mol-1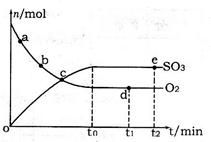
 cC�����Ϊ2L�������н��з�Ӧ���ڢ����ϵ�и����ʵ�����ʱ��仯����������ͼ��ʾ��
cC�����Ϊ2L�������н��з�Ӧ���ڢ����ϵ�и����ʵ�����ʱ��仯����������ͼ��ʾ��
 CO2(g)+ H2(g)����֪CO(g)��H2O(g)����ʼŨ�Ⱦ�Ϊ2 mol��L-1�����ⶨ�÷�Ӧ�ڸ��¶��µ�ƽ�ⳣ��K =1�����жϣ�
CO2(g)+ H2(g)����֪CO(g)��H2O(g)����ʼŨ�Ⱦ�Ϊ2 mol��L-1�����ⶨ�÷�Ӧ�ڸ��¶��µ�ƽ�ⳣ��K =1�����жϣ�