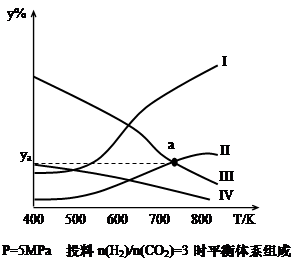��Ŀ����
��Դ����������������ٵ��ش���⣬�״���һ�ֿ�������Դ�����п�����Ӧ�õĹ���ǰ�����о��״�������Ҫ���塣
��1����CO�ϳɼ״��ķ�ӦΪ��CO(g)+2H2(g)  CH3OH(g)���ݻ�Ϊ1L�����ܱ������зֱ����1molCO��2molH2��ʵ���ü״������ʵ������¶ȡ�ʱ��Ĺ�ϵ������ͼ��ʾ���������Ӧ�ġ�H_______0�����������������=�������жϵ�������______��
CH3OH(g)���ݻ�Ϊ1L�����ܱ������зֱ����1molCO��2molH2��ʵ���ü״������ʵ������¶ȡ�ʱ��Ĺ�ϵ������ͼ��ʾ���������Ӧ�ġ�H_______0�����������������=�������жϵ�������______��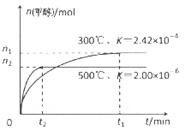
��2�����ù�ҵ�����е�CO2����ȡ�״����䷴ӦΪ��CO2+3H2 CH3OH+H2O��
CH3OH+H2O��
�ٳ��³�ѹ����֪���з�Ӧ�������仯����ͼ��ʾ��
�ɶ�����̼�������Ʊ��״����Ȼ�ѧ����ʽΪ_______��
��Ϊ̽����CO2����ȼ�ϼ״��ķ�Ӧԭ�����ֽ�������ʵ�飺��һ���º����ܱ������У�����1molCO2��3molH2������������Ӧ�����CO2����CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ���ӷ�Ӧ��ʼ��ƽ�⣬v(H2)="_______" �����¶��µ�ƽ�ⳣ����ֵK=______����ʹƽ����ϵ��n(CH3OH)/n(CO2))����Ĵ�ʩ��_______����дһ������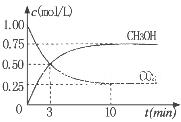
��3����ҵ�����ü״��Ʊ������ij��÷��������֡�
�ټ״���������������Ҫ��ӦΪ��CH3OH(g)  CO(g)+2H2(g)�����ݻ�Ϊ2.0L���ܱ������г���0. 60 molCH3OH(g)����ϵѹǿΪP1����һ�������´ﵽƽ��ʱ����ϵѹǿΪP2����P2/P1 =2.2�����������CH3OH ��ƽ��ת����Ϊ______ ��
CO(g)+2H2(g)�����ݻ�Ϊ2.0L���ܱ������г���0. 60 molCH3OH(g)����ϵѹǿΪP1����һ�������´ﵽƽ��ʱ����ϵѹǿΪP2����P2/P1 =2.2�����������CH3OH ��ƽ��ת����Ϊ______ ��
�ڼ״���������������һ���¶�����Ag/CeO2��ZnOΪ����ʱԭ���������Է�Ӧ��ѡ���ԣ�ѡ����Խ��ʾ���ɵĸ�����Խ�ࣩӰ���ϵ��ͼ��ʾ����n(O2)��n(CH3OH) =0.25ʱ��CH3OH��O2��������Ҫ��Ӧ����ʽΪ______ �����Ʊ�H2��ʱ��ÿ���n(O2))/n(CH3OH)=______��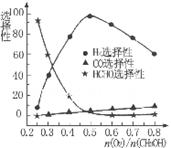
��1�����¶����ߣ�ƽ��ʱ�״��������٣�ƽ�������ƶ���������Ӧ���ȣ����¶����ߣ�ƽ�ⳣ����С��ƽ�������ƶ���������Ӧ���ȣ�����2���� CO2(g)+3H2(g)=CH3OH(l)+H2O(l) ��H="-50KJ/mol." ��0.225mol/(L��min) 5.3 �����¶ȣ����ѹ������H2������H2O��������ϵ�з���ȣ�����3�� ��60�� ��2CH3OH+O2 ="2HCHO+" 2H2O 0. 5
���������������1������Ӧ�ﵽƽ������������¶ȣ�n(CH3OH)��С��ƽ��ʱCH3OH�ĺ������ͣ�˵�������¶ȣ���ѧƽ�����淴Ӧ�����ƶ�������ƽ���ƶ�ԭ���������¶ȣ���ѧƽ�������ȷ�Ӧ�����ƶ����淴Ӧ���������ȷ�Ӧ����������Ӧ�Ƿ��ȷ�Ӧ���ʡ�H��0.��2�� ����ͼһ��֪��CO2(g)+H2(g)=CO(g)+H2O(l) ��H="41KJ/mol," ��ͼ����֪��CO(g)+2H2(g)  CH3OH(g) ��H= -91KJ/mol.����ʽ��ӿɵã�CO2(g) +3H2(g)=CH3OH(l)+H2O(l) ��H="-50KJ/mol." �� V(CO2)=" (1.00-0.25)" mol/L��10min=" 0.075mol/(l��min)." V(H2):V(CO2)=3:1,����V(H2)="3" V(CO2)=" 0.225mol/(L��min)" . �ڸ��¶��µ�ƽ�ⳣ����ֵ
CH3OH(g) ��H= -91KJ/mol.����ʽ��ӿɵã�CO2(g) +3H2(g)=CH3OH(l)+H2O(l) ��H="-50KJ/mol." �� V(CO2)=" (1.00-0.25)" mol/L��10min=" 0.075mol/(l��min)." V(H2):V(CO2)=3:1,����V(H2)="3" V(CO2)=" 0.225mol/(L��min)" . �ڸ��¶��µ�ƽ�ⳣ����ֵ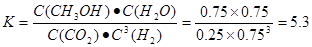 ���ڷ�Ӧ CO2(g) +3H2(g)= CH3OH(l)+H2O(l) ��H=-50KJ/mol.������Ӧ��һ�����ȷ�Ӧ�����Խ����¶���ʹƽ����ϵ��n(CH3OH)/n(CO2)������������ѹ������H2������ˮ�����ӻ�����з�������ȴ�ʩҲ��ʹƽ����ϵ��n(CH3OH)/n(CO2))����3����Ӧ��ʼʱn(CH3OH)=0.6mol,n(CO)=0mol,n(H2)=0mol.���跴Ӧ������CH3OH�ı�����ʵ���ΪX����ﵽƽ��ʱ�����ʵ����ʵ���Ϊn(CH3OH)=" (0.6-X)mol," n(CO) ="Xmol" n(H2)=2Xmol,��������̶����ܱ������е����巴Ӧ��˵����Ӧǰ���ѹǿ�ȵ������ǵ����ʵ����ıȡ�����(0.6+2X)��0.6=2.2,���X=0.36.����CH3OH��ƽ��ת����Ϊ0.36��0.6��100��=60��������ͼ��֪��n(O2)��n(CH3OH) =0.25ʱ�õ��IJ����Ǽ�ȩ��CH3OH��O2��������Ҫ��Ӧ����ʽΪ2CH3OH +O2="2HCHO+" 2H2O�����Ʊ�H2ʱ������n(O2)��n(CH3OH) =0.5ʱѡ������ߣ�������ÿ���n(O2))/n(CH3OH)= 0.5��
���ڷ�Ӧ CO2(g) +3H2(g)= CH3OH(l)+H2O(l) ��H=-50KJ/mol.������Ӧ��һ�����ȷ�Ӧ�����Խ����¶���ʹƽ����ϵ��n(CH3OH)/n(CO2)������������ѹ������H2������ˮ�����ӻ�����з�������ȴ�ʩҲ��ʹƽ����ϵ��n(CH3OH)/n(CO2))����3����Ӧ��ʼʱn(CH3OH)=0.6mol,n(CO)=0mol,n(H2)=0mol.���跴Ӧ������CH3OH�ı�����ʵ���ΪX����ﵽƽ��ʱ�����ʵ����ʵ���Ϊn(CH3OH)=" (0.6-X)mol," n(CO) ="Xmol" n(H2)=2Xmol,��������̶����ܱ������е����巴Ӧ��˵����Ӧǰ���ѹǿ�ȵ������ǵ����ʵ����ıȡ�����(0.6+2X)��0.6=2.2,���X=0.36.����CH3OH��ƽ��ת����Ϊ0.36��0.6��100��=60��������ͼ��֪��n(O2)��n(CH3OH) =0.25ʱ�õ��IJ����Ǽ�ȩ��CH3OH��O2��������Ҫ��Ӧ����ʽΪ2CH3OH +O2="2HCHO+" 2H2O�����Ʊ�H2ʱ������n(O2)��n(CH3OH) =0.5ʱѡ������ߣ�������ÿ���n(O2))/n(CH3OH)= 0.5��
���㣺������ڼ״�ȼ�ϵ�صĻ�ѧ��Ӧԭ�����Ʒ���֪ʶ��

 ������ҵ����ν�����������ϵ�д�
������ҵ����ν�����������ϵ�д�������������Ӧ���ɰ�����ƽ�ⳣ�����±���
| | N2+3H2 2NH3 2NH3 | |||
| �¶� | 25�� | 200�� | 400�� | 600�� |
| ƽ�ⳣ��K | 5��108 | 650 | 0.507 | 0.01 |
��1����ҵ�Ϻϳɰ����¶�һ�������500�棬ԭ���� ��
��2����2 L�ܱ������м���1 mol������3 mol���������й�ҵ�ϳɰ���ģ��ʵ�飬��2���Ӻ�������ѹǿΪԭ����0.8������0��2���ӣ������ķ�Ӧ����Ϊ________mol/(L��min)��
��3������˵���ܱ����÷�Ӧ�ﵽƽ�����________
A�������ƽ�����������ٱ仯 B���ܱ������ڵ�ѹǿ���ٱ仯
C��v (N2) =" 2" v (NH3) D��������ܶȲ��ٱ仯
��4�����д�ʩ�����ܼӿ�÷�Ӧ�ķ�Ӧ���ʣ���������ת���ʵ���______________
A��ʹ�ô��� B����С������� C����߷�Ӧ�¶� D������NH3
��5�������£��ڰ�ˮ�м���һ�������Ȼ�茶��壬����˵���������______��
A����Һ��pH���� B����ˮ�ĵ���ȼ�С C��c(OH-)��С D��c(NH4+)��С
��6������ˮ�������Ũ�ȵ������ϣ�����������ʹc(NH4+)��c(Cl��)��ֵ������________
A�� ��������Ȼ�� B��ͨ�������Ȼ���
C�� ������Һ�¶� D����������������������
ij�о�С���һԪ�л�����HA���ܼ�����ˮ�Ļ����ϵ�е��ܽ�̶Ƚ����о�����25��ʱ������HA��ˮ�в��ֵ��룬��HAŨ��Ϊ ʱ��������Ϊ0.20������ȣ��ѵ����HA������/��ʼHA���ܷ����������ڱ��в��ַ���˫�ۣ����ɣ�HA��2����ƽ����ϵ�У�һԪ�л�����HA���ܼ�����B����ˮ��W���еķ���ϵ��ΪK��K��C��HA��B��C��HA��W��1.0�����ﵽƽ����Է�����ʽ���ڵ�HA�ڱ���ˮ�����ܼ��еı���Ϊ1��1��������Ϣ���£�
ʱ��������Ϊ0.20������ȣ��ѵ����HA������/��ʼHA���ܷ����������ڱ��в��ַ���˫�ۣ����ɣ�HA��2����ƽ����ϵ�У�һԪ�л�����HA���ܼ�����B����ˮ��W���еķ���ϵ��ΪK��K��C��HA��B��C��HA��W��1.0�����ﵽƽ����Է�����ʽ���ڵ�HA�ڱ���ˮ�����ܼ��еı���Ϊ1��1��������Ϣ���£�
| 25��ƽ����ϵ | ƽ�ⳣ�� | �ʱ� | ��ʼ��Ũ�� |
��ˮ�У�HA 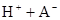 | 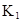 | 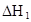 |  |
�ڱ��У�2HA 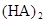 | 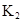 | 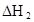 | 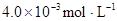 |
�ش��������⣺
��1������25��ʱˮ��Һ��HA�ĵ���ƽ�ⳣ��K1��___________��
��2��25�棬��ˮ��Һ��pHΪ___________������֪��1g2��0.3��lg3��0.5���ڱ���ϵ��HA��ת����Ϊ___________��
��3���ڱ��У�HA�������ۣ�2HA
 ��HA��2����Ӧ�ڽϵ��¶����Է����У���
��HA��2����Ӧ�ڽϵ��¶����Է����У���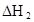 ___________0��
___________0����4��25������ϵ�У�HA�ڱ��з������ۣ������ijʱ����Һ����Ũ������
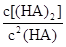 ��130����Ӧ��___________������С�
��130����Ӧ��___________������С� ��4�֣���һ���Ϊ10L�ܱյ������У�ͨ��һ������CO��H2O��g������850��ʱ�������·�Ӧ��CO(g)+H2O(g)  CO2(g)+H2(g) ��H��0
CO2(g)+H2(g) ��H��0
��1��CO��H2OŨ�ȱ仯��ͼ����0��4 min��ƽ����Ӧ���ʦ�(CO)��_______ mol/��L��min������ʱ�÷�Ӧ��ƽ�ⳣ��Ϊ �� 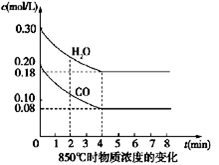
��2����������Щ���������ٷ����仯ʱ������������Ӧ�Ѵﵽƽ��״̬���� ��
| A����������ѹǿ |
| B�����������ܶ� |
| C��CO�����ʵ���Ũ�� |
| D���ܱ������зų����� |
��6�֣��������ƣ�NaClO2����һ��ǿ������Ư�����㷺���ڷ�֯��ӡȾ��ʳƷ��ҵ�����ڼ��Ի������ȶ����ڡ�ijͬѧ�������Ϻ��������NaClO2����Ҫ�������¡�
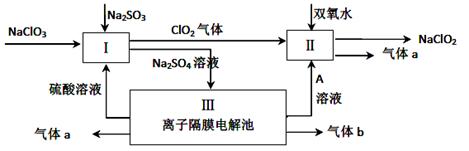
��3����ƽ���з�Ӧ����ʽ ClO3-+ H++ SO32-== ClO2��+ SO42-+
��4��A�Ļ�ѧʽ�� ������������a�ĵ缫��Ӧʽ ��
������һ����Ҫ�Ļ�����Ʒ����������Ρ����صȵ�ԭ�ϡ���ҵ�ϳɰ��ķ�Ӧ����:N2(g) +3H2(g)  2NH3(g) ��H=һ92. 4 KJ��mol-1
2NH3(g) ��H=һ92. 4 KJ��mol-1
��1��2NH3(g)  N2(g) +3H2(g)�ں����ܱ������дﵽƽ��ı�־��
N2(g) +3H2(g)�ں����ܱ������дﵽƽ��ı�־��
�ٵ�λʱ��������3n mol H2:ͬʱ����2n mol NH3����NH3��N2��H2��ʾ��Ӧ���ʱ�Ϊ2��1��3 �ۻ��������ܶȲ��ٸı� �ܻ������ѹǿ���ٸı� �ݻ������ƽ����Է����������ٸı�
| A���٢ۢ� | B���٢ڢܢ� | C���٢ܢ� | D���ڢۢ� |
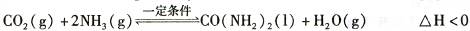
t��ʱ�����ݻ��㶨Ϊ2L���ܱ������м���0.10 molCO:��0. 40 molNH3 ,70 min��ʼ�ﵽƽ�⡣��Ӧ��CO2 ( g)�����ʵ�����ʱ��仯���±���ʾ:
| ʱ�䣯min | ��0 | 30 | 70 | 80 | 100 |
| n(CO2) ��mol | 0.10 | 0.060 | 0.040 | 0.040 | 0.040 |
��20 minʱ������(CO2 )_����������80 minʱ������(H2O)(�>������=����<��)��
����100 minʱ�����������������䣬���������г���0. 050 mo1CO2��0. 20 molNH3�����½���ƽ���CO2��ת������ԭƽ����Ƚ�_ (����������䡱��С��)��
���������淴Ӧ��ƽ�ⳣ��Ϊ_ (������λС��)��
�ܸ��ݱ���������ͼ���л��Ƴ���t����NH3��ת������ʱ��仯��ͼ��;����������������;��(t+10)������ȷ��ͼ������� (��ͼ���еġ�A����B��)��
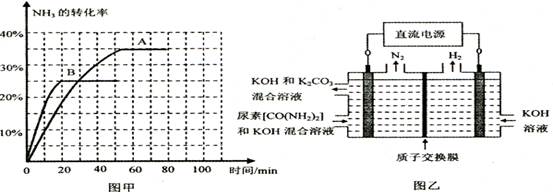
��ͼ����ʾװ��(����������Ϊ���Ե缫)�����ڵ�����ء�CO(NH2)2���ļ�����Һ��ȡ��������װ���������ĵ缫��ӦʽΪ �����������ռ�������22. 4L(��
��)�������ĵ�����Ϊ g(����������ܽ�)��
 H2��+I2
H2��+I2
 CaSO4��2H2O(s)+2 OH��
CaSO4��2H2O(s)+2 OH��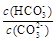 ��С
��С 2C(g)
2C(g) 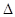 H=" -a" kJ/mol(a��0)����һ���д����Ĺ̶��ݻ����ܱ������м���2molA2��1molB2����500���dz�ַ�Ӧ�ﵽƽ���C��Ũ��Ϊw mol/L���ų�����b kJ��
H=" -a" kJ/mol(a��0)����һ���д����Ĺ̶��ݻ����ܱ������м���2molA2��1molB2����500���dz�ַ�Ӧ�ﵽƽ���C��Ũ��Ϊw mol/L���ų�����b kJ�� CH3CH2OH(g)+3H2O(g) ��Q��Q��0��
CH3CH2OH(g)+3H2O(g) ��Q��Q��0��