��Ŀ����
13��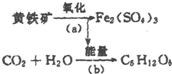 ��ϸ��ұ��������ijЩϸ���������л���ܿ��ɽ�����ʯ��������Һ������������˾������ÿ����е���������������Ҫ�ɷ�ΪFeS2������ΪFe2��SO4��3����ʹ��Һ������ǿ���������ͼ��
��ϸ��ұ��������ijЩϸ���������л���ܿ��ɽ�����ʯ��������Һ������������˾������ÿ����е���������������Ҫ�ɷ�ΪFeS2������ΪFe2��SO4��3����ʹ��Һ������ǿ���������ͼ����1��д�����̣�a���Ļ�ѧ��Ӧ����ʽ��4FeS2+15O2+2H2O$\frac{\underline{\;ϸ��\;}}{\;}$2Fe2��SO4��3+2H2SO4��
��2�����ǻ��������ض���ϸ������Fe2��SO4��3��Һ���������ܽ�ͭ��ʯ��Cu2S�����õ�����������Һ��������Һ�м���������м�õ�ͭ����д�����������е����ӷ�Ӧ����ʽ��
��Cu2S+10Fe3++4H2O=2Cu2++10Fe2++8H++SO42-
��Fe+Cu2+=Fe2++Cu
��Fe+2H+=Fe2++H2��
��3�����в����ڡ�ϸ��ұ�𡱵��ŵ����C����д��ĸ����
A����ƶ��β��Ŀ��ɸ��м�ֵ
B����ϸ���������£�������FeSO4��S���ٴα���������ΪFe2��SO4��3��H2SO4��Fe2��SO4��3��ѭ��ʹ��
C������ϸ����Դ�㷺���������ҵ������ģ����
D���ܴ����Դ���ģ������ڼ�����Ⱦ
��4����ҵ�Ͽ����ô�ͭ����Zn��Ag��Au�ȣ�������Ƶô�ͭ�����ͭ�����������У���ͭ�ӵ�Դ���������������������ͭ�ϵĵ缫��ӦʽΪCu2++2e-=Cu��
��5��ij������������4��ԭ����ƽ��ÿ������b mol��ͭ�����谢���ӵ�����ΪN��ÿ�����Ӵ�����Ϊe C���������ƽ������ǿ��Ϊ2bNeA���ô���ʽ��ʾ����
���� ��1����ϸ�������£��������������������������������
��2������ͭ����������ϸ�������·���������ԭ��Ӧ�����������ӡ�ͭ���ӡ������Ӻ���������ӣ�
����ԭͭ��������ͭ���������ӣ����������ӷ�Ӧ�����������Ӻ�������
��3��A��ϸ��ұ���ƶ��β��Ŀ��ɺ��м�ֵ��
B����ϸ���������£�������FeSO4��S�����л�ԭ�ԣ��ܱ�����������������
C��ϸ��ұ��ʹ�õ��Ǿ�������ܵ�ϸ����
D��ϸ��ұ���ܽ�����Դ���ҷ�Ӧ�жԻ�����Ⱦ��С��
��4����ͭұ��ʱ����ͭ����������ͭ��������
��5��ij������������4��ԭ����ƽ��ÿ������b mol��ͭ����ƽ��ÿ��ת�Ƶ������ʵ���Ϊ2bmol��ת�Ƶ��Ӹ���Ϊ2bN��ÿ�����ӵĵ�����e��ÿ��ͨ�����ӵ���Q=2bNe������ǿ��I=$\frac{Q}{t}$��
��� �⣺��1����ϸ�������£��������������������������������ᣬ��Ӧ����ʽΪ4FeS2+15O2+2H2O$\frac{\underline{\;ϸ��\;}}{\;}$2Fe2��SO4��3+2H2SO4��
�ʴ�Ϊ��4FeS2+15O2+2H2O$\frac{\underline{\;ϸ��\;}}{\;}$2Fe2��SO4��3+2H2SO4��
��2������ͭ����������ϸ�������·���������ԭ��Ӧ�����������ӡ�ͭ���ӡ������Ӻ���������ӣ�
����ԭͭ��������ͭ���������ӣ����������ӷ�Ӧ�����������Ӻ��������漰�����ӷ���ʽΪCu2S+10Fe3++4H2O=2Cu2++10Fe2++8H++SO42-��Fe+Cu2+=Fe2++Cu��Fe+2H+=Fe2++H2����
�ʴ�Ϊ��Cu2S+10Fe3++4H2O=2Cu2++10Fe2++8H++SO42-��Fe+Cu2+=Fe2++Cu��Fe+2H+=Fe2++H2����
��3��A��ϸ��ұ����Ҫ����������Զ�ƶ��β��Ŀ��ɺ��м�ֵ������ȷ��
B����ϸ���������£�������FeSO4��S�����л�ԭ�ԣ��ױ���������������ΪFe2��SO4��3��H2SO4����Fe2��SO4��3��ѭ��ʹ�ã�����ȷ��
C��ϸ��ұ��ʹ�õ��Ǿ�������ܵ�ϸ�������������е�ϸ�����иù��ܣ��ʴ���
D��ϸ��ұ���ܽ�����Դ���ҷ�Ӧ�жԻ�����Ⱦ��С�������ܴ����Դ���ģ������ڼ�����Ⱦ
������ȷ��
��ѡC��
��4����ͭұ��ʱ����ͭ����������ͭ�����������Դ�ͭ���ӵ�Դ������������ӦʽΪCu2++2e-=Cu���ʴ�Ϊ������Cu2++2e-=Cu��
��5��ij������������4��ԭ����ƽ��ÿ������b mol��ͭ����ƽ��ÿ��ת�Ƶ������ʵ���Ϊ2bmol��ת�Ƶ��Ӹ���Ϊ2bN��ÿ�����ӵĵ�����e��ÿ��ͨ�����ӵ���Q=2bNe������ǿ��I=$\frac{Q}{t}$=$\frac{2bNe}{1}$A=2bNeA��
�ʴ�Ϊ��2bNe��
���� �����Խ���ұ��Ϊ���忼��������ԭ��Ӧ�����ӷ���ʽ����д��֪ʶ�㣬Ϊ��Ƶ���㣬��ȷ�������ʼ����ԭ���ǽⱾ��ؼ����ѵ��ǵ���ǿ�ȵļ��㣮

| A�� | ��״���£�22.4 L�����й��ۼ���ĿΪ19NA | |
| B�� | 12.4g�������к��е�P-P������0.1NA | |
| C�� | 2mol SO2��1mol O2�����V2O5���ڵ������£��ܱ������м��ȷ�Ӧ�����������ʵķ���������2NA | |
| D�� | ��NO2��N2O4���ӹ�NA����������״���£������Ϊ22.4 L |
| A�� | �ռ�Ȼ��ơ���������ˮ | B�� | ����ռ�ɱ���Һ̬�Ȼ��� | ||
| C�� | ����ʯ��ˮ�����ᱵ��ʯī | D�� | ���ᡢ̼�ᡢ������Һ�� |
| A�� | Na+��H+��MnO4-��SO42- | B�� | Na+��K+��NO3-��SO42- | ||
| C�� | Fe2+��Cl-��CO32-��SiO32- | D�� | Mg2+��Na+��Cl-��HCO3- |
| A�� | ����ԭ�ӵ����ԭ������Ϊ12a/b | |
| B�� | Wg����ԭ�ӵ����ʵ���ΪW/aNA mol | |
| C�� | Wg ����ԭ��������������Ϊ 10W/a�� | |
| D�� | ��Ԫ�ص�Ħ������ΪaNA g/mol |
| A�� | ����ʵ�ˮ��Һһ���ܵ��� | |
| B�� | �ǵ���ʵ�ˮ��Һһ�����ܵ��� | |
| C�� | �ᡢ���������ڵ���ʣ����������ﶼ�Ƿǵ���� | |
| D�� | ���������ˮ��Һ�������״̬�������ܵ���������Ӻ������Ӷ�����Ļ����� |

 ��ͼ��ʾ��ij��ѧ��ȤС����Ƶ�Ȥζʵ��װ��ͼ��ͼ��A��D��Ϊ̼����B������CΪ���������üס��������е���Һ��ǰ����ȡ����д�����B��ʵ��ʱ�Ų�����Һ�У�
��ͼ��ʾ��ij��ѧ��ȤС����Ƶ�Ȥζʵ��װ��ͼ��ͼ��A��D��Ϊ̼����B������CΪ���������üס��������е���Һ��ǰ����ȡ����д�����B��ʵ��ʱ�Ų�����Һ�У�