��Ŀ����
8��ʵ������Ҫ0.1mol•L-1��NaOH��Һ450mL��������������ش��������⣺��1��ʵ�����г���������ƽ���ձ���Կ�����Ҫ�����������в�������500mL����ƿ�ͽ�ͷ�ιܣ�
��2�����ݼ����֪������Ҫ��NaOH������Ϊ2.0g��
��3�����в���ʹ������ҺŨ��ƫ�����C������ĸ����ͬ����ƫС����BDEF����Ӱ�����A��
A������ƿδ���������������Һ
B������ʱ��������ˮ�����̶��ߣ����ý�ͷ�ι�����
C��NaOH���ձ����ܽ��δ��ȴ������ת�Ƶ�����ƿ��
D��������ƿת��ʱ������Һ�彦��
E��δϴ���ܽ�NaOH���ձ�
F������ʱ���ӿ̶���
��4����ͼ�Ǹ�ѧ����ʵ�������Ƹ�NaOH��Һ�ù���ʾ��ͼ���ݴ˻ش��������⣺

�۲�����NaOH��Һ�Ĺ���ʾ��ͼ��ָ�������д�����Ǣ٢ۢݣ��������ţ���
���� ��1����������һ�����ʵ���Ũ�ȵ���Һ�IJ����dz������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�Ⱥ�װƿ�����������������
��2������ʵ������450mL����ƿ����Ӧѡ��500mL����ƿ�����������m=CVM���ݴ˼��㣻
��3���������������n��V��Ӱ�죬����c=����������������ҺŨ�ȵ�Ӱ�죻
��4����������һ�����ʵ���Ũ����Һ����ȷ�����������������ȷʹ�÷������
��� �⣺��1������ʵ������450mL����ƿ����Ӧѡ��500mL����ƿ����������һ�����ʵ���Ũ�ȵ���Һ�IJ����dz������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�Ⱥ�װƿ��֪����������У�������ƽ��ҩ�ס��ձ�����������500mL����ƿ�ͽ�ͷ�ιܣ��ʳ���������ƽ���ձ���ҩ���⣬����Ҫ��������500mL����ƿ�ͽ�ͷ�ιܣ��ʴ�Ϊ����������500mL����ƿ�ͽ�ͷ�ιܣ�
��2������ʵ������450mL����ƿ����Ӧѡ��500mL����ƿ�������Ƴ�500mL��Һ��������������ƹ��������m=CVM=0.1mol/L��0.5L��40g/mol=2.0g���ʴ�Ϊ��2.0g��
��3��A������ƿδ���������������Һ����Ũ����Ӱ�죻
B������ʱ��������ˮ�����̶��ߣ����ý�ͷ�ι��������������IJ�ֹ��ˮ���������ʣ���Ũ��ƫС��
C��NaOH���ձ����ܽ��δ��ȴ������ת�Ƶ�����ƿ�У������ȴ����Һ���ƫС��Ũ��ƫ�ߣ�
D��������ƿת��ʱ������Һ�彦�����ᵼ�����ʵ���ʧ����Ũ��ƫС��
E��δϴ���ܽ�NaOH���ձ����ᵼ�����ʵ���ʧ����ҺŨ��ƫС��
F������ʱ���ӿ̶��ߣ�����Һ���ƫ��Ũ��ƫС��
�ʴ�Ϊ��C��BDEF��A��
��4������ͲΪ��ȡ���������������ܽ����ʣ��ʢٴ���
���ò�����������ٹ�����ܽ⣬�ʢ���ȷ��
�۲�������������ʱ���������¶�Ӧ��������ƿ�̶����·����ʢ۴���
�ܼ�ˮ���̶��ߵ��·���������ȷ���ʢ���ȷ��
�ݶ���ʱ���۾�Ӧƽ�ӿ̶��ߣ��ʢݴ���
�Ӹ�ҡ�ȣ�ʹ��Һ��Ͼ��ȣ�������ȷ���ʢ���ȷ��
�ʴ�Ϊ���٢ۢݣ�
���� ���⿼����һ�����ʵ���Ũ�ȵ����ƣ�Ҫע�����ʵ����ʡ�ͬʱҪ��ס�������裬�淶������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�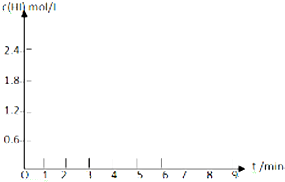 ��0.8mol I2��g����1.2mol H2��g������ij1L�ܱ������У���һ���¶��·�����Ӧ��I2��g��+H2��g��?2HI��g�����ﵽƽ�⣮HI�����������ʱ��ı仯�������ʾ��
��0.8mol I2��g����1.2mol H2��g������ij1L�ܱ������У���һ���¶��·�����Ӧ��I2��g��+H2��g��?2HI��g�����ﵽƽ�⣮HI�����������ʱ��ı仯�������ʾ��| HI������� | 1min | 2min | 3min | 4min | 5min | 6min | 7min |
| ����I | 26% | 42% | 52% | 57% | 60% | 60% | 60% |
| ����II | 20% | 33% | 43% | 52% | 57% | 65% | 65% |
��2��������I�ӿ�ʼ��Ӧ������ƽ��ʱ��H2�ķ�Ӧ����Ϊ0.12 mol/��L•min����
��3��Ϊ�ﵽ����II�����ݣ����ڷ�Ӧ��ϵ���ܸı�IJ����ǽ����¶ȣ�
��4���÷�Ӧ�ġ�H��0�����������������=����
��5��������I�´ﵽƽ�����7minʱ���������ѹ��Ϊԭ����һ�룮����ͼ�л���c��HI����ʱ��仯�����ߣ�
| A�� | 1 mol����������Ϊ32 g | |
| B�� | ���³�ѹ�£�1 mol CO2�������ԼΪ22.4 L | |
| C�� | CO2��Ħ������Ϊ44 g•mol-1 | |
| D�� | 1 L 2 mol•L-1��BaCl2��Һ�к�Cl-�ĸ���Ϊ2.408��1024 |
| A�� |  �������� | B�� |  ���� | C�� |  ���� | D�� |  ���Ͻ� |
| A�� | �����Ħ��������98g | B�� | 1mol N2������Ϊ28g/mol | ||
| C�� | Ħ�������ʵ����ĵ�λ | D�� | 1mol������������16g |
| A�� | 1.02 | B�� | 1.68 | C�� | 1.00 | D�� | 0.986 |
4NH3��g��+5O2��g��?4NO��g��+6H2O��g��������������ȷ���ǣ�������
| A�� | ����λʱ��������xmolNO��ͬʱ����x molNH3����Ӧ�ﵽƽ��״̬ | |
| B�� | ����������ѹǿ���ٸı䣬��˵����Ӧ�Ѵﵽƽ��״̬ | |
| C�� | �����������ܶȲ��ٸı䣬��˵����Ӧ�Ѵﵽƽ��״̬ | |
| D�� | �ﵽ��ѧƽ��������������ݻ�������Ӧ���ʼ�С���淴Ӧ�������� |
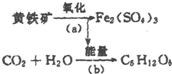 ��ϸ��ұ��������ijЩϸ���������л���ܿ��ɽ�����ʯ��������Һ������������˾������ÿ����е���������������Ҫ�ɷ�ΪFeS2������ΪFe2��SO4��3����ʹ��Һ������ǿ���������ͼ��
��ϸ��ұ��������ijЩϸ���������л���ܿ��ɽ�����ʯ��������Һ������������˾������ÿ����е���������������Ҫ�ɷ�ΪFeS2������ΪFe2��SO4��3����ʹ��Һ������ǿ���������ͼ��