��Ŀ����
��֪��101 kPa�£�1 g��������������ȫȼ��������̬ˮʱ���ų�����120.9 kJ.������˵������ȷ����(����)
| A��������ȼ����Ϊ241.8 kJ/mol |
| B��1 mol H2O(l)�����������1 mol H2O(g)������� |
| C����Ӧ���Ȼ�ѧ����ʽΪ��2H2(g)��O2(g)===2H2O(g)����H����483.6 kJ/mol |
| D��2 mol H2(g)��1 mol O2(g)���е������ܺʹ���2 mol H2O(g)���е����� |
D
���������ȼ��������һ�������£�1mol��ȼ����ȫȼ�������ȶ���������ʱ���ų���������A����ȷ��ˮ���ȶ�������Һ̬��1 g��������������ȫȼ��������̬ˮʱ���ų�����120.9 kJ������1mol������ȫ������̬ˮʱ�ų���������120.9 kJ��2��241.8 kJ���÷�Ӧ�Ƿ��ȷ�Ӧ����˵��2 mol H2(g)��1 mol O2(g)���е������ܺʹ���2 mol H2O(g)���е�������D��ȷ��ѡ��BC����ȷ����ѡD��
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣���������ǿ����ע�ض�ѧ������֪ʶ���̺�ѵ����ͬʱ�����ض�ѧ��������������ּ������ѧ�����������ɡ��ܽ����������������������ѧ������˼ά������Ӧ��������

��ϰ��ϵ�д�
�����Ŀ
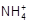 )��c(Cl��)��c(H+)��c(OH��)
)��c(Cl��)��c(H+)��c(OH��)