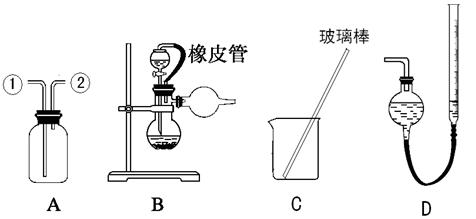
��Ŀ����
���к͵ζ����ⶨ�ռ�Ĵ��ȣ����ռ��к����������������õĿ��������ʣ��Ը���ʵ��ش�
��1��ȷ��ȡ4.1g�ռ���Ʒ��
��2������Ʒ���250mL����Һ����Ҫ��������С�ձ����������� ����ͷ�ιܡ�
��3���� ��ȡ10mL����Һ�� �У����������ƣ������μӼ��μ�����ָʾ����
��4����0.2010 mol��L����������ζ������ռ���Һ���ζ�ʱ ����ת ʽ�ζ��ܵIJ��������� �ֲ�ͣ��ҡ����ƿ������ע����ƿ����Һ��ɫ�ı仯��ֱ���ζ��յ㣬�ζ��յ�ʱ��ƿ����Һ��PHԼΪ ���ﵽ�յ�ľ��������ǣ� ��
��5��������ʵ��ζ����������±���
���ϱ����ݣ���������ռ���Һ��Ũ�ȣ� ��
(6)�������������ݣ������ռ�Ĵ��ȣ�
��7���ζ�ʱ���ζ��ܼ��첿�ֵζ�ǰ�����ݣ��ζ����������ݣ��ⶨ�����_______����ƫ�ߡ�ƫ�ͻ���Ӱ�죬������������ȷ����
��1��ȷ��ȡ4.1g�ռ���Ʒ��
��2������Ʒ���250mL����Һ����Ҫ��������С�ձ����������� ����ͷ�ιܡ�
��3���� ��ȡ10mL����Һ�� �У����������ƣ������μӼ��μ�����ָʾ����
��4����0.2010 mol��L����������ζ������ռ���Һ���ζ�ʱ ����ת ʽ�ζ��ܵIJ��������� �ֲ�ͣ��ҡ����ƿ������ע����ƿ����Һ��ɫ�ı仯��ֱ���ζ��յ㣬�ζ��յ�ʱ��ƿ����Һ��PHԼΪ ���ﵽ�յ�ľ��������ǣ� ��
��5��������ʵ��ζ����������±���
| �ζ����� | ����Һ�����mL�� | �����������mL�� | |
| �ζ�ǰ������mL�� | �ζ��������mL�� | ||
| ��һ�� | 10.00 | 0.50 | 20.4 |
| �ڶ��� | 10.00 | 4.00 | 24.1 |
���ϱ����ݣ���������ռ���Һ��Ũ�ȣ� ��
(6)�������������ݣ������ռ�Ĵ��ȣ�
��7���ζ�ʱ���ζ��ܼ��첿�ֵζ�ǰ�����ݣ��ζ����������ݣ��ⶨ�����_______����ƫ�ߡ�ƫ�ͻ���Ӱ�죬������������ȷ����
[(1)~(4)С��ÿ��1�֣�����2 �ֹ�15��]
��2��250mL����ƿ ��3����ʽ�ζ��� ��ƿ
��4������ң�3.1��4.4�� ��Һ�ɻ�ɫ��Ϊ��ɫ���Ұ���Ӳ���ɫ����2 �֣�
��5��0.4020 mol��L���� ��6��98.05% ��7��ƫ��
��2��250mL����ƿ ��3����ʽ�ζ��� ��ƿ
��4������ң�3.1��4.4�� ��Һ�ɻ�ɫ��Ϊ��ɫ���Ұ���Ӳ���ɫ����2 �֣�
��5��0.4020 mol��L���� ��6��98.05% ��7��ƫ��
��2��ȷ����һ�����ʵ����ǵ���Һʱ��Ҫ��������250ml����ƿ��
��3�����������Ǽ��Ҫ��ʽ�ζ��ܣ�����ҺҪ������ƿ�С�
��4�������к͵ζ��Ļ���ʵ����������ȵı�ɫ��Χ��3.1��4.4�������յ�ʱ����������Һ
�ɻ�ɫ��Ϊ��ɫ���Ұ���Ӳ���ɫ��
��5���������ĵ����������19.90ml��20.10mol������ƽ��ֵ��20.00ml�������������Ƶ�Ũ
����0.2010 mol��L������0.020L��0.010L��0.4020 mol��L������
��6����Ʒ���������Ƶ�������0.4020 mol��L������0.25L��40g/mol��4.020g�����Դ�����4.020g
��4.1g��100����98.05%��
��7��������ʧ���൱����������ƫ�����Բⶨ���ƫ�ߡ�
��3�����������Ǽ��Ҫ��ʽ�ζ��ܣ�����ҺҪ������ƿ�С�
��4�������к͵ζ��Ļ���ʵ����������ȵı�ɫ��Χ��3.1��4.4�������յ�ʱ����������Һ
�ɻ�ɫ��Ϊ��ɫ���Ұ���Ӳ���ɫ��
��5���������ĵ����������19.90ml��20.10mol������ƽ��ֵ��20.00ml�������������Ƶ�Ũ
����0.2010 mol��L������0.020L��0.010L��0.4020 mol��L������
��6����Ʒ���������Ƶ�������0.4020 mol��L������0.25L��40g/mol��4.020g�����Դ�����4.020g
��4.1g��100����98.05%��
��7��������ʧ���൱����������ƫ�����Բⶨ���ƫ�ߡ�

��ϰ��ϵ�д�
 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
�����Ŀ
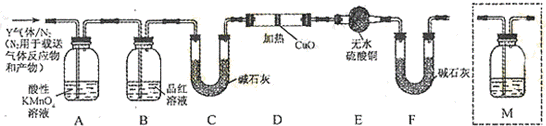
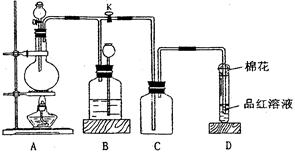

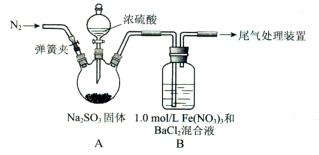
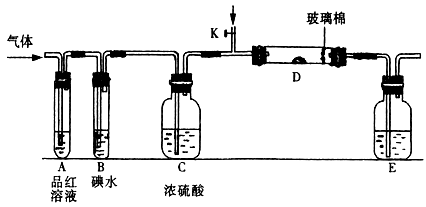
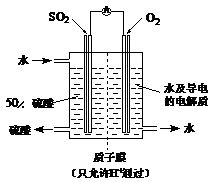
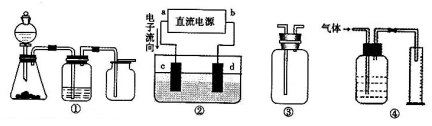
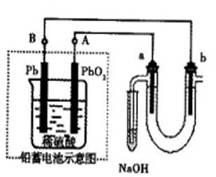
 ij�о���С�����A-D������װ�ã������ظ�ʹ�ã�����й�ʵ��
ij�о���С�����A-D������װ�ã������ظ�ʹ�ã�����й�ʵ��