��Ŀ����
��15�֣�ij�о�С��̽��SO2��Fe(NO3)3��Һ�ķ�Ӧ��
��֪��1.0 mol/L��Fe(NO3)3��Һ��pH=l
��ش�
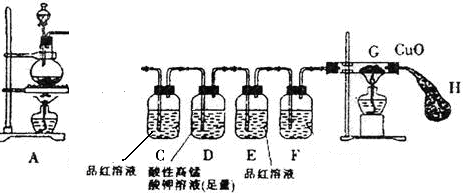
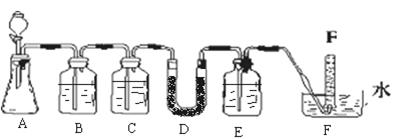
��1��װ��A�з�Ӧ�Ļ�ѧ����ʽ��____________��
��2��Ϊ�ų�������ʵ��ĸ��ţ��μ�Ũ����֮ǰӦ���еIJ�����___________��
��3��װ��B�в����˰�ɫ��������ɷ���____________��˵��SO2����________�ԡ�
��4������B�в�����ɫ������ԭ��
�۵�1��____________��
�۵�2��SO2��Fe3+��Ӧ��
�۵�3��������������SO2��NO3����Ӧ��
�ٰ��۵�2��װ��B�з�Ӧ�����ӷ���ʽ��___________��Ϊ֤���ù۵�Ӧ��һ���������ɵ������ʣ���ʵ�������������__________��
�ڰ��۵�3��ֻ�轫װ��B�е�Fe(NO3)3��Һ�滻Ϊ�������������Һ������ͬ�����½���ʵ�顣Ӧѡ����Լ��ǣ�����ţ�____________��
a��0��1 mol/Lϡ����
b��1��5 mol/L Fe(NO3)2��Һ
c��6��0 mol/L NaNO3��0��2 mol/L����������ϵ���Һ

��֪��1.0 mol/L��Fe(NO3)3��Һ��pH=l
��ش�
��1��װ��A�з�Ӧ�Ļ�ѧ����ʽ��____________��
��2��Ϊ�ų�������ʵ��ĸ��ţ��μ�Ũ����֮ǰӦ���еIJ�����___________��
��3��װ��B�в����˰�ɫ��������ɷ���____________��˵��SO2����________�ԡ�
��4������B�в�����ɫ������ԭ��
�۵�1��____________��
�۵�2��SO2��Fe3+��Ӧ��
�۵�3��������������SO2��NO3����Ӧ��
�ٰ��۵�2��װ��B�з�Ӧ�����ӷ���ʽ��___________��Ϊ֤���ù۵�Ӧ��һ���������ɵ������ʣ���ʵ�������������__________��
�ڰ��۵�3��ֻ�轫װ��B�е�Fe(NO3)3��Һ�滻Ϊ�������������Һ������ͬ�����½���ʵ�顣Ӧѡ����Լ��ǣ�����ţ�____________��
a��0��1 mol/Lϡ����
b��1��5 mol/L Fe(NO3)2��Һ
c��6��0 mol/L NaNO3��0��2 mol/L����������ϵ���Һ
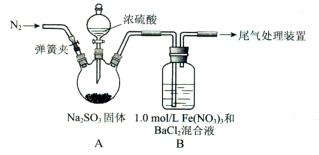
��1��Na2SO3+H2SO4(Ũ) = Na2SO4+ SO2��+H2O��2�֣�
��2�����ɼУ���װ����ͨ��һ��ʱ���N2���رյ��ɼУ�2�֣�
��3��BaSO4��1�֣� ��ԭ�ԣ�1�֣�
��4���۵�1��SO2��Fe3+������������NO3������Ӧ��2�֣�
��SO2+ 2Fe3++Ba2++2H2O="=" BaSO4��+2Fe2++4H+��2�֣�
ȡ����B��Һ���Թ��У���������������KMnO4��Һ����ɫ��ȥ����Һ����Fe2+��Ȼ����ʪ��ĵ���KI��ֽ����B����ĩ�ˣ�����������˵��NO3��û��Ӧ��֤���۵�2������3�֣�
��c��2�֣�
��2�����ɼУ���װ����ͨ��һ��ʱ���N2���رյ��ɼУ�2�֣�
��3��BaSO4��1�֣� ��ԭ�ԣ�1�֣�
��4���۵�1��SO2��Fe3+������������NO3������Ӧ��2�֣�
��SO2+ 2Fe3++Ba2++2H2O="=" BaSO4��+2Fe2++4H+��2�֣�
ȡ����B��Һ���Թ��У���������������KMnO4��Һ����ɫ��ȥ����Һ����Fe2+��Ȼ����ʪ��ĵ���KI��ֽ����B����ĩ�ˣ�����������˵��NO3��û��Ӧ��֤���۵�2������3�֣�
��c��2�֣�
��1��Ũ������������Ʒ�Ӧ����SO2������ʽΪNa2SO3+H2SO4(Ũ) = Na2SO4+ SO2��+H2O��
��2��Ҫ�ų�������ʵ��ĸ��ţ������Ҫ�ž�װ���еĿ����������õ������ž�װ���еĿ����������ɼУ���װ����ͨ��һ��ʱ���N2���رյ��ɼС�
��3��B����Һ�����ԣ���˲������������ᱵ������ֻ�������ᱵ��������˵��SO2��������������������ģ����SO2���л�ԭ�ԡ�
��4�����ݹ۵�2��3��֪���۵�1Ӧ��SO2��Fe3+������������NO3������Ӧ��
������Ǣڣ��������ӵĻ�ԭ�������������ӣ����Է���ʽΪO2+ 2Fe3++Ba2++2H2O==
BaSO4��+2Fe2++4H+��Ҫ�����������ӣ��������仹ԭ�Խ��м��飬��ȡ����B��Һ���Թ��У���������������KMnO4��Һ����ɫ��ȥ����Һ����Fe2+�����NO3��������SO2�����仹ԭ������NO��NO���������ԣ���������ʪ��ĵ���KI��ֽ������ʪ��ĵ���KI��ֽ����B����ĩ�ˣ�����������˵��NO3��û��Ӧ��֤���۵�2������
��Ϊ�ų������ӵĸ��ţ�����ѡ�������ӵ������μ��ɣ����Դ�ѡC��
��2��Ҫ�ų�������ʵ��ĸ��ţ������Ҫ�ž�װ���еĿ����������õ������ž�װ���еĿ����������ɼУ���װ����ͨ��һ��ʱ���N2���رյ��ɼС�
��3��B����Һ�����ԣ���˲������������ᱵ������ֻ�������ᱵ��������˵��SO2��������������������ģ����SO2���л�ԭ�ԡ�
��4�����ݹ۵�2��3��֪���۵�1Ӧ��SO2��Fe3+������������NO3������Ӧ��
������Ǣڣ��������ӵĻ�ԭ�������������ӣ����Է���ʽΪO2+ 2Fe3++Ba2++2H2O==
BaSO4��+2Fe2++4H+��Ҫ�����������ӣ��������仹ԭ�Խ��м��飬��ȡ����B��Һ���Թ��У���������������KMnO4��Һ����ɫ��ȥ����Һ����Fe2+�����NO3��������SO2�����仹ԭ������NO��NO���������ԣ���������ʪ��ĵ���KI��ֽ������ʪ��ĵ���KI��ֽ����B����ĩ�ˣ�����������˵��NO3��û��Ӧ��֤���۵�2������
��Ϊ�ų������ӵĸ��ţ�����ѡ�������ӵ������μ��ɣ����Դ�ѡC��

��ϰ��ϵ�д�
�����Ŀ
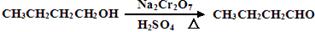 ����Ӧ��Ͳ������������б����£�
����Ӧ��Ͳ������������б����£�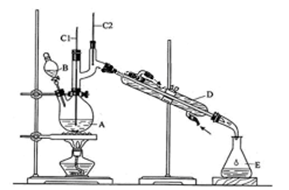
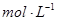 NaOH��Һ�У��ж�п����ȫ��Ӧ�ı�־�ǣ�__________�����ȡ����Ƭ��ˮ��ϴ����ɺ������������Ϊm2����п�Ʋ㵥����Ϊh��п���ܶ�Ϊ
NaOH��Һ�У��ж�п����ȫ��Ӧ�ı�־�ǣ�__________�����ȡ����Ƭ��ˮ��ϴ����ɺ������������Ϊm2����п�Ʋ㵥����Ϊh��п���ܶ�Ϊ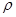 ����h=__________
����h=__________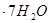 �����IJ����ǣ���1g�̷�����30mL����ˮ�ܽⲢ����H2SO4��Һ��H3PO4��Һ������0.02mol/L KMnO4����Һ�ζ�����Һ�ձ�ɷۺ�ɫ��ֹͣ�ζ������ı���ҺVmL����Ӧ���漰����Ҫ��ѧ����ʽ�У�
�����IJ����ǣ���1g�̷�����30mL����ˮ�ܽⲢ����H2SO4��Һ��H3PO4��Һ������0.02mol/L KMnO4����Һ�ζ�����Һ�ձ�ɷۺ�ɫ��ֹͣ�ζ������ı���ҺVmL����Ӧ���漰����Ҫ��ѧ����ʽ�У�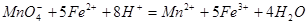
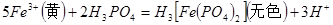
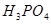 ��������__________��
��������__________��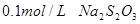 ��Һ��
��Һ��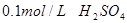 ���ձ�����ˮ����ˮ�������
���ձ�����ˮ����ˮ�������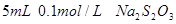 ��
��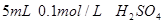 ��
��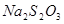 ��
��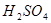 ��Ӧ�Ļ�ѧ����ʽΪ ��
��Ӧ�Ļ�ѧ����ʽΪ ��