��Ŀ����
����Ŀ����ʯ�����������������й㷺����;����ش�
��1����ʯ���н���Ԫ�ص�ԭ�ӽṹʾ��ͼ��_____��
��2��������ʯ����Һ���Ȼ�����Һ��ϡ����IJ�����������__________��
��3����ʯ�ҽ���ˢǽ�ڣ������ǽ���Ӳ����Ӧ�Ļ�ѧ����ʽ��_____��
��4��ij�������������ԣ������ᣩ����ѡ����ʯ������������Ӧ�Ļ�ѧ����ʽ��_________��
��5������ʯ�ҷ����ľ�Ұ�һ��������Ͽ��Ƶø�Ч����ũҩ���ڰۡ���ʹ��ʱ��ѡ������¶ ˮ���糿���ѡ��ڰۡ�����ֲ�ᆬҶ�ϣ��������ɼ���塣
�١��ڰۡ�����ʯ�Ҹ���Ч�������������˼��Ը�ǿ�����ʣ��÷�Ӧ�Ļ�ѧ����ʽ��________��
�ڡ��ڰۡ��п��ṩֲ�������Ϳ�������Ӫ��Ԫ����_____����Ԫ�ط��ţ���
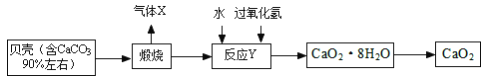
���𰸡� ![]() �ֱ�ȡ������Ʒ��Һ���Թ��У������еμӼ���ʯ����Һ��������ϡ���ᣬ����������ʯ����Һ�������Ա仯�����Ȼ�����Һ CO2+Ca(OH)2�TCaCO3��+H2O Ca(OH)2+H2SO4�TCaSO4+2H2O Ca(OH)2+K2CO3=CaCO3��+2KOH K
�ֱ�ȡ������Ʒ��Һ���Թ��У������еμӼ���ʯ����Һ��������ϡ���ᣬ����������ʯ����Һ�������Ա仯�����Ȼ�����Һ CO2+Ca(OH)2�TCaCO3��+H2O Ca(OH)2+H2SO4�TCaSO4+2H2O Ca(OH)2+K2CO3=CaCO3��+2KOH K
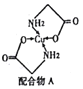
��������(1)��ʯ���н���Ԫ��Ϊ�ƣ��Ƶ�ԭ�ӽṹʾ��ͼΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(2)��ʯ����Һ�Լ��ԡ��Ȼ�����Һ�����ԡ�ϡ���������ԣ�����������Һ�IJ���������Ϊ���ֱ�ȡ������Ʒ��Һ���Թ��У������еμӼ���ʯ����Һ��������ϡ���ᣬ����������ʯ����Һ�������Ա仯�����Ȼ�����Һ���ʴ�Ϊ���ֱ�ȡ������Ʒ��Һ���Թ��У������еμӼ���ʯ����Һ��������ϡ���ᣬ����������ʯ����Һ�������Ա仯�����Ȼ�����Һ��
(3)ʯ�ҽ��е���Ҫ�ɷ����������ƣ���������еĶ�����̼��Ӧ����̼��ƺ�ˮ��̼�����һ�ֲ�����ˮ�İ�ɫ�ļ�Ӳ�����ʣ������ǽ���üȰ���Ӳ����Ӧ�Ļ�ѧ����ʽΪ��CO2+Ca(OH)2�TCaCO3��+H2O���ʴ�Ϊ��CO2+Ca(OH)2�TCaCO3��+H2O��
(4)�������������ᷴӦ��������ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ��Ca(OH)2+H2SO4�TCaSO4+2H2O���ʴ�Ϊ��Ca(OH)2+H2SO4�TCaSO4+2H2O��
(5)������������̼��ط�Ӧ����̼��ƺ��������أ���ѧ����ʽΪCa(OH)2+K2CO3=CaCO3��+2KOH���ʴ�Ϊ��Ca(OH)2+K2CO3=CaCO3��+2KOH��
��ֲ����Ҫ��Ӫ��Ԫ����N��P��K�����ڰ����к���KԪ�����ʴ�Ϊ��K��