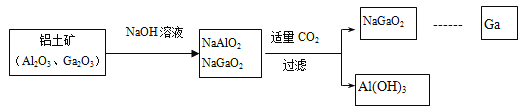
ЬтФПФкШн
ЁОЬтФПЁПвбжЊ25ЁцЁЂ101 kPaЪБФГаЉЮяжЪЕФШМЩеШШЪ§ОнШчЯТЃК
УћГЦ | ЛЏбЇЪН | ІЄH (kJ/mol) | УћГЦ | ЛЏбЇЪН | ІЄH (kJ/mol) |
ЪЏФЋ | C(s) | 393.5 | ввЭщ | C2H6(g) | 1560 |
Н№ИеЪЏ | C(s) | 395.0 | ввЯЉ | C2H4(g) | 1411 |
ЧтЦј | H2(g) | 285.8 | ввШВ | C2H2(g) | 1300 |
вЛбѕЛЏЬМ | CO(g) | 283.0 | ввДМ | C2H5OH(l) | 1367 |
ЃЈ1ЃЉЯрЭЌжЪСПЕФЩЯЪіАЫжжЮяжЪЃЌЭъШЋШМЩеЗХШШзюЖрЕФУћГЦЪЧ____ЁЃ
ЃЈ2ЃЉЪЏФЋгыН№ИеЪЏЛЅЮЊ____ЁЃ
a.ЭЌЮЛЫи b.ЭЌЫивьаЮЬх c.ЭЌЗжвьЙЙЬх d.ЭЌЯЕЮя
ГЃЮТГЃбЙЯТЃЌЖўепИќЮШЖЈЕФЪЧ___ЁЃЃЈЬюжаЮФУћГЦЃЉ
ЃЈ3ЃЉдкБъзМзДПіЯТЃЌ22.4LCOКЭC2H2ЕФЛьКЯЦјЬхдкЙ§СПЕФбѕЦјжаЭъШЋШМЩеЗХГі1096.6 kJЕФШШЃЌдђШМЩеВњЩњЕФCO2ЕФЮяжЪЕФСПЮЊ___molЁЃ
ЃЈ4ЃЉЙРЫудкNiзїДпЛЏМСЪБЃЌввЯЉгыЧтЦјЗЂЩњМгГЩЗДгІЕФьЪБфЁЃC2H4(g)+H2(g)=C2H6(g) ІЄH=____ЁЃ
ЃЈ5ЃЉвбжЊЦЦЛЕЯТСаЛЏбЇМќашвЊЮќЪеЕФФмСПШчЯТЃК
CЃC | C=C | HЃH |
347kJ/mol | 619kJ/mol | 436kJ/mol |
ИљОнЃЈ4ЃЉЕФНсТлЃЌЙРЫуCЃHМќЕФМќФмЮЊ____kJ/molЁЃ
aЃЎ322.5 bЃЎ422.5 c.522.5 dЃЎ622.5
ЁОД№АИЁПЧтЦј b ЪЏФЋ 1.8 Ѓ137kJ/mol b
ЁОНтЮіЁП
ЃЈ1ЃЉЯрЭЌжЪСПЕФПЩШМЮяЃЌЯрЖдЗжзгжЪСПдНаЁЃЌЮяжЪЕФСПдНЖрЃЌдйНсКЯЗДгІШШБШНЯЗХГіЕФШШСПДѓаЁЃЛ
ЃЈ2ЃЉЪЏФЋгыН№ИеЪЏЪЧЭЌжждЊЫизщГЩЕФВЛЭЌЕЅжЪЃЛФмСПдНЕЭдНЮШЖЈЃЛ
ЃЈ3ЃЉЩшCOЕФЮяжЪЕФСПЮЊx molЃЌНсКЯШШСПЪ§ОнСаЗНГЬЧѓГіВЮМгЗДгІЕФCOгыC2H2ИїздЕФЮяжЪЕФСПЃЌдйРћгУдЊЫиЪиКуевГіЩњГЩЕФЖўбѕЛЏЬМЕФЮяжЪЕФСПЃЛ
ЃЈ4ЃЉвРОнИЧЫЙЖЈТЩЙЙдьвбжЊШШЛЏбЇЗНГЬЪНЃЌевГіЗДгІШШжЎМфЕФЙиЯЕЃЛ
ЃЈ5ЃЉЗДгІШШ=ЗДгІЮяЖЯМќЫљЮќЪеЕФШШСП-ЩњГЩЮяГЩМќЫљЗХГіЕФШШСПЁЃ
ЃЈ1ЃЉНсКЯБэИёЪ§ОнБШНЯЯрЭЌжЪСПЕФЩЯЪіАЫжжЮяжЪЃЌЧтЦјЕФЮяжЪЕФСПзюДѓЃЌЭъШЋШМЩеЗХШШзюЖрЃЌЙЪД№АИЮЊЃКЧтЦјЃЛ
ЃЈ2ЃЉЪЏФЋгыН№ИеЪЏЛЅЮЊЭЌЫивьаЮЬхЃЛЪЏФЋБШН№ИеЪЏЕФФмСПЕЭЃЌЪЏФЋИќЮШЖЈЃЌЙЪД№АИЮЊЃКbЃЛЪЏФЋЃЛ
ЃЈ3ЃЉдкБъзМзДПіЯТЃЌ22.4L COКЭC2H2ЕФЛьКЯЦјЬхЕФЮяжЪЕФСПЮЊ![]() =1 molЃЌЩшCOЕФЮяжЪЕФСПЮЊx molЃЌдђИљОнЩЯЪіБэИёЪ§ОнПЩжЊЃЌ283.0 kJ/mol
=1 molЃЌЩшCOЕФЮяжЪЕФСПЮЊx molЃЌдђИљОнЩЯЪіБэИёЪ§ОнПЩжЊЃЌ283.0 kJ/mol![]() x mol+1300 kJ/mol
x mol+1300 kJ/mol![]() (1-x)mol=1096.6 kJЃЌНтЕУx=0.2 molЃЌдђCOЕФЮяжЪЕФСПЮЊ0.2 molЃЌC2H2ЕФЮяжЪЕФСПЮЊЃЈ1-0.2ЃЉmol=0.8 molЃЌЩњГЩЖўбѕЛЏЬМЕФЮяжЪЕФСПЮЊ0.2mol+0.8mol
(1-x)mol=1096.6 kJЃЌНтЕУx=0.2 molЃЌдђCOЕФЮяжЪЕФСПЮЊ0.2 molЃЌC2H2ЕФЮяжЪЕФСПЮЊЃЈ1-0.2ЃЉmol=0.8 molЃЌЩњГЩЖўбѕЛЏЬМЕФЮяжЪЕФСПЮЊ0.2mol+0.8mol![]() 2=1.8 molЃЌЙЪД№АИЮЊЃК1.8ЃЛ
2=1.8 molЃЌЙЪД№АИЮЊЃК1.8ЃЛ
ЃЈ4ЃЉИљОнБэИёаХЯЂПЩжЊЃЌввЯЉЕФШМЩеШШЮЊ1411 kJ/molЃЌдђШШЛЏбЇЗНГЬЪНЮЊЃКC2H4(g)+3O2(g)=2CO2(g)+2H2O(l) ![]() = 1411 kJ/molЂйЃЌЭЌРэИљОнЧтЦјЕФШМЩеШШКЭввЭщЕФШМЩеШШПЩжЊЕУЃЌH2(g)+
= 1411 kJ/molЂйЃЌЭЌРэИљОнЧтЦјЕФШМЩеШШКЭввЭщЕФШМЩеШШПЩжЊЕУЃЌH2(g)+![]() O2(g)= H2O(l)
O2(g)= H2O(l) ![]() = 285.8 kJ/molЂкЃЌC2H6(g)+
= 285.8 kJ/molЂкЃЌC2H6(g)+![]() O2(g)=2CO2(g)+3H2O(l)
O2(g)=2CO2(g)+3H2O(l) ![]() = 1560 kJ/molЂлЃЌдђЂй+Ђк-ЂлЕУC2H4(g)+H2(g)=C2H6(g) ІЄH=1411 kJ/mol285.8 kJ/mol-ЃЈ1560 kJ/molЃЉ
= 1560 kJ/molЂлЃЌдђЂй+Ђк-ЂлЕУC2H4(g)+H2(g)=C2H6(g) ІЄH=1411 kJ/mol285.8 kJ/mol-ЃЈ1560 kJ/molЃЉ![]() Ѓ137kJ/molЃЌЙЪД№АИЮЊЃКЃ137kJ/molЃЛ
Ѓ137kJ/molЃЌЙЪД№АИЮЊЃКЃ137kJ/molЃЛ
ЃЈ4ЃЉЩшCЃHМќЕФМќФмЮЊx kJ/molЃЌИљОнБэИёЪ§ОнПЩЕУЃЌ619 kJ/mol+4x kJ/mol+436kJ/mol-(347kJ/mol+6x kJ/mol)= Ѓ137kJ/molЃЌНтЕУx=422.5ЃЌbЯюе§ШЗЃЌЙЪД№АИЮЊЃКbЁЃ

 дФЖСПьГЕЯЕСаД№АИ
дФЖСПьГЕЯЕСаД№АИЁОЬтФПЁПЙЄвЕЩЯгУЩСаППѓЃЈжївЊГЩЗжЮЊZnSЃЌЛЙКЌгаCdSЁЂFe2O3ЕШдгжЪЃЉЮЊдСЯЩњВњZnSO4ЁЄ7H2OЕФЙЄвеСїГЬШчЯТЃКЃЈвбжЊCdЕФН№ЪєЛюЖЏадНщгкZnКЭFeжЎМфЃЉ
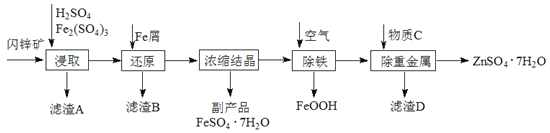
ЃЈ1ЃЉДгТЫдќAжаПЩЛёЕУвЛжжЕЛЦЩЋЗЧН№ЪєЕЅжЪЕФИБВњЦЗЃЌЦфЛЏбЇЪНЮЊ________ЁЃ
ЃЈ2ЃЉНўШЁЙ§ГЬжаFe2(SO4)3ЕФзїгУЪЧ_______________ЃЌНўШЁЪБFe2(SO4)3гыZnSЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЮЊ____________________________________________ЁЃ
ЃЈ3ЃЉГ§ЬњЙ§ГЬПижЦШмвКЕФpHдк5.4зѓгвЃЌИУЗДгІЕФРызгЗНГЬЪНЮЊ_______________________ЁЃИУЙ§ГЬдкПеЦјШыПкДІЩшМЦСЫвЛИіРрЫЦСмдЁХчЭЗЕФзАжУЃЌЦфФПЕФЪЧ____________________________________ЁЃ
ЃЈ4ЃЉжУЛЛЗЈГ§жиН№ЪєРызгЪЧCd2+ЃЌЫљгУЮяжЪCЮЊ_________ЁЃ
ЃЈ5ЃЉСђЫсаПЕФШмНтЖШгыЮТЖШжЎМфЕФЙиЯЕШчЯТБэЃК
ЮТЖШ/Ёц | 0 | 20 | 40 | 60 | 80 | 100 |
ШмНтЖШ/g | 41.8 | 54.1 | 70.4 | 74.8 | 67.2 | 60.5 |
ДгГ§жиН№ЪєКѓЕФСђЫсаПШмвКжаЛёЕУСђЫсаПОЇЬхЕФЪЕбщВйзїЮЊ__________ЁЂ__________ЁЂЙ§ТЫЁЂИЩдяЁЃ
ЁОЬтФПЁПЯТСаИїзщЮяжЪжаЃЌВЛТњзузщФкШЮвтСНжжЮяжЪдквЛЖЈЬѕМўЯТОљФмЗЂЩњЗДгІЕФЪЧ
| Мз | вв | Бћ |
A | Al | HCl | NaOH |
B | NH3 | O2 | HNO3 |
C | SiO2 | NaOH | HF |
D | SO2 | Ca(OH)2 | NaHCO3 |
A.AB.BC.CD.D