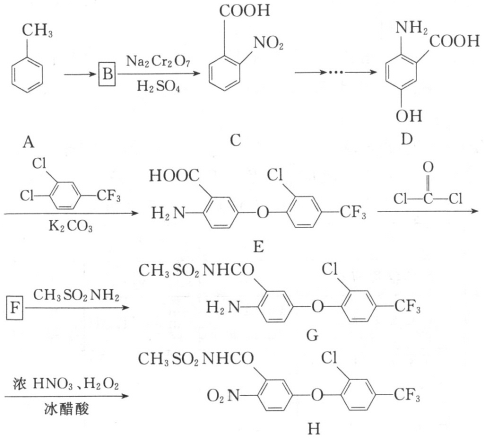
��Ŀ����
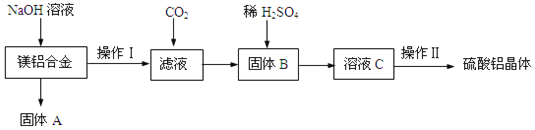
����Ŀ����ҵ������п����Ҫ�ɷ�ΪZnS��������CdS��Fe2O3�����ʣ�Ϊԭ������ZnSO4��7H2O�Ĺ����������£�����֪Cd�Ľ�����Խ���Zn��Fe֮�䣩
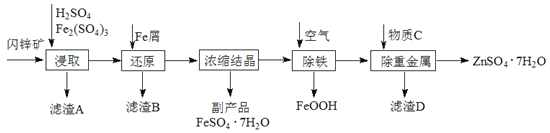
��1��������A�пɻ��һ�ֵ���ɫ�ǽ������ʵĸ���Ʒ���仯ѧʽΪ________��
��2����ȡ������Fe2(SO4)3��������_______________����ȡʱFe2(SO4)3��ZnS������Ӧ�Ļ�ѧ����ʽΪ____________________________________________��
��3���������̿�����Һ��pH��5.4���ң��÷�Ӧ�����ӷ���ʽΪ_______________________���ù����ڿ�����ڴ������һ��������ԡ��ͷ��װ�ã���Ŀ����____________________________________��
��4���û������ؽ���������Cd2+����������CΪ_________��
��5������п���ܽ�����¶�֮��Ĺ�ϵ���±���
�¶�/�� | 0 | 20 | 40 | 60 | 80 | 100 |
�ܽ��/g | 41.8 | 54.1 | 70.4 | 74.8 | 67.2 | 60.5 |
�ӳ��ؽ����������п��Һ�л������п�����ʵ�����Ϊ__________��__________�����ˡ����
���𰸡�S �������� Fe2(SO4)3+ZnS=ZnSO4+2FeSO4+S�� 4Fe2++O2+6H2O=4FeOOH��+8H+ �����������Һ�ĽӴ�������ӿ췴Ӧ���� Zn��п�� 60������������Ũ�� ���½ᾧ
��������
��1����п�����Ҫ�ɷ�ΪZnS��������CdS��Fe2O3�����ʣ�����ϡ�����������������ӦZnS+Fe2��SO4��3=ZnSO4+2FeSO4+S����CdS+Fe2��SO4��3=CdSO4+2FeSO4+S����Fe2O3+3H2SO4=Fe2��SO4��3+3H2O���ʴ�����A�л�õ�һ�ֵ���ɫ�ǽ�������ΪS��
��2����ȡʱ��������������Ԫ�صĻ��ϼ�Ϊ-2�ۣ�����ǿ��ԭ�ԣ���������Fe3+���н�ǿ�������ԣ���������������������п����������Ӧ������������������п��S�����ݵ�ʧ�����غ㡢ԭ���غ㣬��ȡʱFe2��SO4��3��ZnS������Ӧ�Ļ�ѧ����ʽΪZnS+Fe2��SO4��3=ZnSO4+2FeSO4+S����
��3��Ũ���ᾧ��ĸҺ���б�������������Һ���������ӱ�����ΪFeOOHʱ����Ԫ�ػ��ϼ���+2����Ϊ+3�ۣ������������������������Ԫ����0�۽�Ϊ-2�ۣ����ݵ�ʧ�����غ㡢����غ㡢ԭ���غ���ƽ����÷�ӦΪ4Fe2++O2+6H2O=4FeOOH��+8H+���ڿ�����ڴ������һ��������ԡ��ͷ��װ�ã�Ŀ���������������Һ�ĽӴ�������ӿ췴Ӧ���ʣ�
��4�������û���Ӧԭ����Ӧѡ���Cd���õĽ��������ݳ���ԭ��ȥCd2+��ͬʱֻ������Zn2+�����������Ľ��������ӣ��������CΪп��Zn����
��5��������֪��0��100�棬����п���ܽ�������¶����߶��������¶����߶���С������60����������п���ܽ�������˴ӳ��ؽ����������п��Һ�л������п�����ʵ�����Ϊ��60����������Ũ������ȴ�ᾧ�����ˡ���������Ʊ�����п���塣

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ����֪25�桢101 kPaʱijЩ���ʵ�ȼ�����������£�
���� | ��ѧʽ | ��H (kJ/mol) | ���� | ��ѧʽ | ��H (kJ/mol) |
ʯī | C(s) | 393.5 | ���� | C2H6(g) | 1560 |
���ʯ | C(s) | 395.0 | ��ϩ | C2H4(g) | 1411 |
���� | H2(g) | 285.8 | ��Ȳ | C2H2(g) | 1300 |
һ����̼ | CO(g) | 283.0 | �Ҵ� | C2H5OH(l) | 1367 |
��1����ͬ�����������������ʣ���ȫȼ�շ�������������____��
��2��ʯī����ʯ��Ϊ____��
a.ͬλ�� b.ͬ�������� c.ͬ���칹�� d.ͬϵ��
���³�ѹ�£����߸��ȶ�����___�������������ƣ�
��3���ڱ�״���£�22.4LCO��C2H2�Ļ�������ڹ�������������ȫȼ�շų�1096.6 kJ���ȣ���ȼ�ղ�����CO2�����ʵ���Ϊ___mol��
��4��������Ni������ʱ����ϩ�����������ӳɷ�Ӧ���ʱ䡣C2H4(g)+H2(g)=C2H6(g) ��H=____��
��5����֪�ƻ����л�ѧ����Ҫ���յ��������£�
C��C | C=C | H��H |
347kJ/mol | 619kJ/mol | 436kJ/mol |
���ݣ�4���Ľ��ۣ�����C��H���ļ���Ϊ____kJ/mol��
a��322.5 b��422.5 c.522.5 d��622.5
����Ŀ��![]() ��Ҫ����ѡ�����αװͿ�ϵ����ϵȡ����ܿ�ʯ
��Ҫ����ѡ�����αװͿ�ϵ����ϵȡ����ܿ�ʯ![]() ��
��![]() ��CoO������
��CoO������![]() ��
��![]() ��
��![]() ��
��![]() ����
����![]() ��������ͼ1��
��������ͼ1��

�±��г��˼������������������������pH![]() ��������ȫ����ָ��Һ������Ũ�ȵ���
��������ȫ����ָ��Һ������Ũ�ȵ���![]()
|
|
|
|
| |
��ʼ������pH |
|
|
|
|
|
������ȫ��pH |
|
|
|
|
|
![]() д������ȡ������
д������ȡ������![]() ������Ӧ�����ӷ���ʽ______��
������Ӧ�����ӷ���ʽ______��
![]() ����ȡ�������
����ȡ�������![]() �⣬�ܿ�ʯ�л��ܱ�
�⣬�ܿ�ʯ�л��ܱ�![]() ��ԭ��������______��
��ԭ��������______��
![]() ��
��![]() ��Ŀ��������
��Ŀ��������![]() ��
��![]() �������������ɵIJ��������______��
�������������ɵIJ��������______��
![]() ��������������
��������������![]() ��Һ����
��Һ����![]() ��Һ��Ҫ
��Һ��Ҫ![]() �����½��У����˵ļ��ȷ�ʽΪ______���¶ȿ�����
�����½��У����˵ļ��ȷ�ʽΪ______���¶ȿ�����![]() ��ԭ��Ϊ______��
��ԭ��Ϊ______��
![]()
![]() ��ʵ�鷽�������������������Һ�м���______������
��ʵ�鷽�������������������Һ�м���______������![]() ��Һ���ܵ�
��Һ���ܵ�![]() ʵ������ʹ���Լ���
ʵ������ʹ���Լ���![]() ��Һ��������ȡ��������
��Һ��������ȡ��������![]() ��
��