��Ŀ����
����Ŀ���������Ƴ���Ư����ɱ���������������������Ʊ��治���������տ�����CO2�����ʡ�
(1)ij����С����̽��ij����������Ʒ�Ƿ��Ѿ����ʣ�ȡ������Ʒ���ܽ⣬����_______________��Һ��������а�ɫ������֤��Na2O2�Ѿ����ʡ�
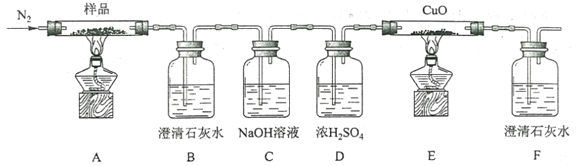
(2)�ÿ���С��Ϊ�˴��Բⶨ�������ƵĴ��ȣ����dz�ȡag��Ʒ�����������ͼװ�� ���ⶨ�������Ƶ�����������

��A�з�����Ӧ���ӷ���ʽΪ___________________
�ڽ��������Ӻ��Ժ�����еĵ�һ��������__________
��д��װ��C�з��������з�Ӧ�Ļ�ѧ����ʽ______________________________��_______________________________________________________
��D��NaOH��Һ������__________________________
�ݶ�����Ͳ��ˮ�����������ɱ�״�������������ΪVmL������Ʒ�й������Ƶ������� ��Ϊ__________________
���𰸡�CaCl2��BaCl2��Һ CaCO3+2H+=Ca2++H2O+CO2�� ���װ�õ������� 2CO2+2Na2O2=2Na2CO3+O2 2Na2O2+2H2O=4NaOH+O2�� ����δ��Ӧ��CO2 ![]()
��������
(1) �������Ʊ��治���������տ�����CO2����̼���ƺ����������������Ʊ��ʣ�����̼���ƣ�
(2)���������������̼��ˮ��Ӧ�ų�������������������������ɼ���������Ƶ��������Ӷ�����������ƵĴ��ȡ�
(1) �������Ʊ��治���������տ�����CO2����̼���ƺ����������������Ʊ��ʣ�����̼���ƣ�̼�������Ȼ��ƻ��Ȼ�����Ӧ����̼��ƻ�̼�ᱵ���������Լ���CaCl2��BaCl2��Һ��������а�ɫ������֤��Na2O2�Ѿ����ʣ�
(2) ��A�д���ʯ�����ᷴӦ�����Ȼ��ơ�������̼��ˮ��������Ӧ�����ӷ���ʽΪCaCO3+2H+=Ca2++H2O+CO2�� ��
�ڽ��������Ӻ��Ժ�����еĵ�һ�������Ǽ��װ�õ������ԣ�
�۽���װ��C�������ж�����̼��ˮ������������̼��������Ʒ�Ӧ����̼���ƺ�������ˮ������������Ʒ�Ӧ�����������ƺ�������������Ӧ�Ļ�ѧ����ʽΪ2CO2+2Na2O2=2Na2CO3+O2 ��2Na2O2+2H2O=4NaOH+O2����
�ܶ�����̼������������Һ��Ӧ����̼���ƺ�ˮ��D��NaOH��Һ������������δ��Ӧ�Ķ�����̼���壻
�����ɱ�״�������������ΪVmL�������������ʵ�����![]() �����ݹ�ϵʽ2Na2O2~~O2����������Ƶ����ʵ�����
�����ݹ�ϵʽ2Na2O2~~O2����������Ƶ����ʵ�����![]() ����Ʒ�й������Ƶ�����Ϊ
����Ʒ�й������Ƶ�����Ϊ![]() ���������Ƶ���������Ϊ
���������Ƶ���������Ϊ![]()
![]() ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�