��Ŀ����
����Ŀ��������ѧ֪ʶ�ش��������⣺
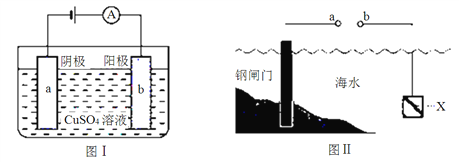
��.������(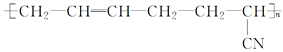 )�������������͡��������ܣ��ϳɶ����Ķ��ֵ�����_________________��_______________����ͨ��___________��Ӧ�õ���
)�������������͡��������ܣ��ϳɶ����Ķ��ֵ�����_________________��_______________����ͨ��___________��Ӧ�õ���
��. ������������ζ���������͵��£����Ƴ����µ�Ȧ���ܷ�����������࣬����Ѫ�ܵȣ������ɵ���������ȹ���(CH3)2SiCl2ˮ��õ����������������ˮ���۳ɵľ۹������پ������Ƴɵģ����û�ѧ����ʽд�������������ȹ���ˮ�⼰�������۵ķ���ʽ_________________��_________________��
���𰸡� CH2=CH-CH=CH2 CH2=CH-CN �Ӿ۷�Ӧ ��CH3��2SiCl2+2H2O����CH3��2Si��OH��2+2HCl n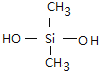 ����n-1��H2O+
����n-1��H2O+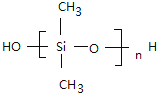
����������.������( )�������������͡��������ܣ��ϳɶ����Ķ��ֵ�����CH2=CH-CH=CH2��CH2=CH-CN����������ͨ���Ӿ۷�Ӧ�õ�����
)�������������͡��������ܣ��ϳɶ����Ķ��ֵ�����CH2=CH-CH=CH2��CH2=CH-CN����������ͨ���Ӿ۷�Ӧ�õ�����
��.�������ȹ���ˮ�⼰�������۵Ļ�ѧ����ʽΪ��CH3��2SiCl2+2H2O����CH3��2Si��OH��2+2HCl��n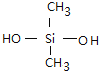 ����n-1��H2O+
����n-1��H2O+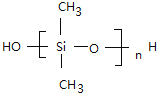 ��
��

 ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�
ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д� ����������ϵ�д�
����������ϵ�д�����Ŀ��ij��ѧ��ȤС���Fe3O4��������Ȥ���Ӳο��������ҵ�������������������ӡ�øߵ�Fe3O4�۵Ĺ������̼�ͼ��
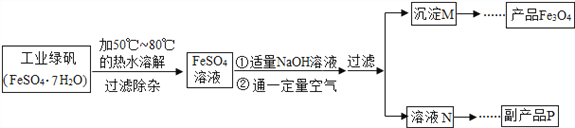 ������������
������������
����һ�����������ڲ�ͬ�¶��µ��ܽ�������ʾ��
�¶�/�� | 0 | 10 | 30 | 50 | 60 | 70 | 80 | 90 |
�ܽ��/g | 14.0 | 17.0 | 25.0 | 33.0 | 35.3 | 33.0 | 30.5 | 27.0 |
���϶���Fe��OH��2������������Ӧԭ��Ϊ��4Fe(OH)2 +2H2O+O2=4Fe(OH)3
�����������Fe3O4�ķ�Ӧԭ��Ϊ��Fe(OH)2 + 2Fe(OH)3 ![]() Fe3O4+4H2O
Fe3O4+4H2O
������������
��1���ܽ�ʱ����50����80������ˮĿ����__________________________________________��
��2������ƷP��___________________________��
��3��Ϊ�˵õ������IJ�Ʒ�����ˡ�ϴ�Ӻ���еľ����������Ϊ______________________________������
�����ȷ����
������ʾ����������������������ʱ�ֽ�������������������ﲻ�䣩��
��Fe2O3��CO��Ӧ�����¶����߶����еģ�������Fe3O4��������FeO����ɫ�����������Fe��
Ϊȷ�������Ϸ����Ƶõ������������Ĵ��ȣ�ͬѧ�dz�ȡ��23.28g��Fe3O4��Ʒ�������ʵ�����̽����
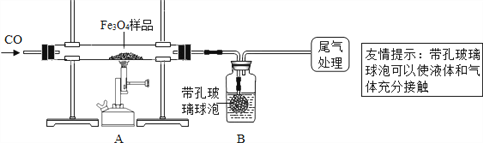
��1������ͬѧ��ͨ������Bװ�÷�Ӧǰ��������仯������ȷ�ϸ�Fe3O4��Ʒ�е�������B�е�����Լ���_______________________������ţ���
A������ʯ��ˮ B����������Ũ��Һ C��ϡ���� D��ˮ
��2������ʵ������У�CO�������Ϊ��Ӧ���⣬�������û��У�
��ʵ�鿪ʼʱ���ž�װ���еĿ�������ֹ����ʱ������ը��
��ֹͣ���Ⱥ���ͨCO���壬��ֹ�����ﱻ�������Լ�B�е���Һ������A�����ҿ���_____________________________�������ʵ��ľ�ȷ����
��3�����������ⶨ�����Ƴ���Ӧ������
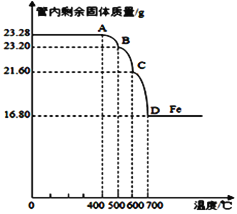
�ٸ�Fe3O4��Ʒ�к��е����ʳɷ�______________________���ѧʽ��
�ڼ�����Ʒ��Fe3O4������������д����Ҫ�ļ�����̣��� _____________