��Ŀ����
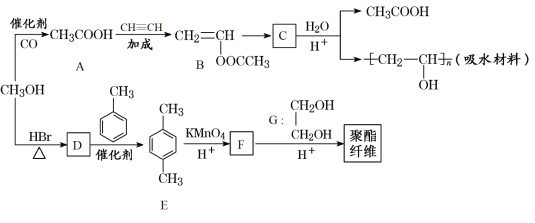
����Ŀ��ij��ˮ���Ϻ;�����ά�ϳ�·����ͼ��ʾ��
�ش��������⣺
(1)B�еĹ�����������_________��
(2)B��C�ķ�Ӧ������____________��
(3)��д����CH3OH����D�Ļ�ѧ����ʽ__________��
(4)E��ͬ���칹��H�������������������ڷ����廯����ں˴Ź�������������壬�ҷ����֮��Ϊ1��1��2��6����H�Ľṹ��ʽΪ___________��
(5)д��������ά�Ľṹ��ʽ��_________��
(6)�����������̣��Ա�����ϩΪ��ʼԭ�ϣ�ѡ�ñ�Ҫ���Լ��ϳɶ������ұ�(![]() )��д���ϳ�·��_______ (�ýṹ��ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ����)����ʾ��������һ���ڼ�λ����ȡ�����ұ�һ������λ���λ����ȡ����
)��д���ϳ�·��_______ (�ýṹ��ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ����)����ʾ��������һ���ڼ�λ����ȡ�����ұ�һ������λ���λ����ȡ����
���𰸡�̼̼˫�������� �Ӿ۷�Ӧ CH3OH+HBr��CH3Br+H2O 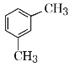
![]() CH2=CH2
CH2=CH2![]() CH3CH2Br
CH3CH2Br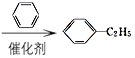
![]()
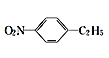
��������
�״����ڴ��������£��״���һ����̼��Ӧ�������ᣬ�������Ȳ�����ӳɷ�Ӧ����CH2=CHOOCCH3��B�����Ӿ۷�Ӧ����C������C��ˮ�����֪��C�Ľṹ��ʽΪ��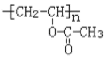 ���״����廯�ⷢ��ȡ����Ӧ����D(1-�����)��D�ͼױ���Ӧ����E��E����������F��F�ͼ״���Ӧ����G����F�ǶԶ������ᣬE�ǶԶ��ױ���1-�����ͺͼױ�����ȡ����Ӧ���ɶԶ��ױ����ݴ˷������
���״����廯�ⷢ��ȡ����Ӧ����D(1-�����)��D�ͼױ���Ӧ����E��E����������F��F�ͼ״���Ӧ����G����F�ǶԶ������ᣬE�ǶԶ��ױ���1-�����ͺͼױ�����ȡ����Ӧ���ɶԶ��ױ����ݴ˷������
(1) B�Ľṹ��ʽΪ��CH2=CHOOCCH3��B�к��еĹ�������̼̼˫����������
(2)���ݷ�����B�����Ӿ۷�Ӧ����C ��B��C�ķ�Ӧ�����ǼӾ۷�Ӧ��
(3)�״����廯�ⷢ��ȡ����Ӧ����D(1-�����)����ѧ����ʽCH3OH+HBr��CH3Br+H2O��
(4) E�ǶԶ��ױ�����ͬ���칹��H�������������������ڷ����廯���˵�����б������ں˴Ź�������������壬�ҷ����֮��Ϊ1��1��2��6��˵���ṹ�����ֲ�ͬ��������ԭ�ӣ���H�Ľṹ��ʽΪ ��
��
(5) F�ǶԶ������ᣬ���Ҷ���������Ӧ���ɾ�����ά���ṹ��ʽΪ��![]() ��
��
(6)��ϩ���廯�ⷢ���ӳɷ�Ӧ���������飬�������뱽��Ӧ�����ұ����ұ���Ũ������������Ũ���ᷴӦ���ɶ������ұ���д���ϳ�·��Ϊ��CH2=CH2![]() CH3CH2Br
CH3CH2Br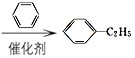
![]()


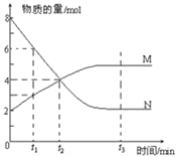
����Ŀ��10��ʱ����NaHCO3������Һ����ø���Һ��pH�������±仯��
�¶�/�� | 10 | 20 | 30 | ������к���ȴ��50�� |
pH | 8.3 | 8.4 | 8.5 | 8.8 |
��1����ͬѧ��Ϊ������Һ��pH���ߵ�ԭ����HCO3-ˮ��̶����ʼ�����ǿ���÷�Ӧ�����ӷ���ʽΪ______________________����ͬѧ��Ϊ����ҺpH���ߵ�ԭ����NaHCO3���ȷֽ⣬������Na2CO3�����ƶ�Na2CO3��ˮ��̶�________NaHCO3(��������������С����)����ͬѧ��Ϊ�ס��ҵ��ж϶�����֣�����Ϊ��
��2��ֻҪ�ڼ�����е���Һ�м����������Լ�X����������������________(����������������)�ж���ȷ���Լ�X��________(��ѡ��)��
A��Ba(OH)2��Һ | B��BaCl2��Һ |
C��NaOH��Һ | D�������ʯ��ˮ |
��3���������Ϸ���NaHCO3�ķֽ��¶�Ϊ150����������________(����������������)�ж��Ǵ���ģ�������________________________________________________��