��Ŀ����
6����һ�����ĩ����ըҩ����������ɣ��仯ѧ�ɷ�Ϊ��ѧ�����ļס������ֻ���������ֵ��ʣ�Ϊ��������ɣ�����������ʵ�飺��ȡ29.14g���壬��һ�ܱ��������������������ұ�ը�����ɵ������ۺϳɱ�״���µ����Ϊ8.96L��������������N2��CO2�������Ϊ1��3��
�ڽ��ٷ�Ӧ��Ĺ����������ˮ�ܽⲢ���ˣ���Һ��ֻ��һ�����α���ͨ�������������ɵ�3.21g����ɫ��������ɫ��Ӧ�������ɫ��
�۲������л�ʣ������2.14g��Ԫ�ط������������к�������Ԫ�أ������мײμӵ�ijһ������Ӧ����������˲��������������NaOH��Һ�У���ʣ1.12g���壬�ù�����һ�ֿɱ����������ĵ��ʶ������ڳ�ʪ�Ŀ������ױ�������
��ش��������⣺
��1���ҵĻ�ѧʽΪKNO3�����ĵ���ʽΪ
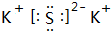 ��
����2��д����ըҩ��ը��Ӧ�Ļ�ѧ����ʽΪS+2KNO3+3C$\frac{\underline{\;\;��\;\;}}{\;}$K2S+N2��+3CO2����
��3��������������Ӧ�Ļ�ѧ����ʽΪ2Al+Fe2O3$\frac{\underline{\;����\;}}{\;}$Al2O3+2Fe�����������������ṩ��ը��Ӧ���������������
��4�����������У��п�����Ϊ�����Ʒ����D��
A��KCl B��K2CO3 C��Na2S D��CuO
��5�����һ��ʵ�鷽����̽�����ڳ�ʪ�Ŀ����б������ļ�̬ȡ�����������ܽ����Һ�ֳ����ݣ�һ�ݵμ����軯����Һ����죬˵����Fe3+����һ�ݼ�K3[Fe��CN��6]�����軯�أ���Һ������ɫ����˵����Fe2+����
���� ��ȡ29.14g���壬��һ�ܱ��������������������ұ�ը�����ɵ������ۺϳɱ�״���µ����Ϊ8.96L��������������N2��CO2�������Ϊ1��3��������������ʵ���=$\frac{8.96L}{22.4L/mol}$=0.4mol������Ϊ$\frac{1}{4}$��0.4mol=0.1mol��������̼Ϊ0.4mol-0.1mol=0.3mol��
�ڽ��ٷ�Ӧ��Ĺ����������ˮ�ܽⲢ���ˣ���Һ��ֻ��һ�����α���ͨ�������������ɵ�3.21g����ɫ��������ɫ����ΪS�������ʵ���=$\frac{3.2g}{32g/mol}$=0.1mol����ɫ��Ӧ����ɫ�ܲ����۲�������ɫ������KԪ�أ��ʱ�ΪK2S�������ʵ���Ϊ0.1mol��
�۲����ڹ��˳�������2.14g��Ԫ�ط������������к�������Ԫ�أ������мײμӵ�ijһ������Ӧ����������˲��������������NaOH��Һ�У���ʣ1.12g���壬�ù�����һ�ֿɱ����������ĵ��ʶ������ڳ�ʪ�Ŀ������ױ���������ΪFe�������ʵ���=$\frac{1.12L}{56g/mol}$=0.02mol���������л�����Al2O3��������=2.14g-1.12g=1.02g�����ʵ���=$\frac{1.02g}{102g/mol}$=0.01mol���������Ϊ�������������ԭ���غ��֪������Fe��Oԭ����Ŀ֮��=0.02��0.01��3=2��3���ʼ�ΪFe2O3��ԭ�����ĩ�к���Al���ʣ�
���Ϸ����������������������ֵ��ʷ�Ӧ����K2S��N2��CO2�����������ԭ��Ӧ��֪���������ֵ���ΪC��S����ԭ���غ��֪�������Һ���K��N��O����Ԫ�أ���K��N��Oԭ����Ŀ֮��=0.1��2��0.1��2��0.3��2=211��3������ΪKNO3���ݴ˽��
��� �⣺��ȡ29.14g���壬��һ�ܱ��������������������ұ�ը�����ɵ������ۺϳɱ�״���µ����Ϊ8.96L��������������N2��CO2�������Ϊ1��3��������������ʵ���=$\frac{8.96L}{22.4L/mol}$=0.4mol������Ϊ$\frac{1}{4}$��0.4mol=0.1mol��������̼Ϊ0.4mol-0.1mol=0.3mol��
�ڽ��ٷ�Ӧ��Ĺ����������ˮ�ܽⲢ���ˣ���Һ��ֻ��һ�����α���ͨ�������������ɵ�3.21g����ɫ��������ɫ����ΪS�������ʵ���=$\frac{3.2g}{32g/mol}$=0.1mol����ɫ��Ӧ����ɫ�ܲ����۲�������ɫ������KԪ�أ��ʱ�ΪK2S�������ʵ���Ϊ0.1mol��
�۲����ڹ��˳�������2.14g��Ԫ�ط������������к�������Ԫ�أ������мײμӵ�ijһ������Ӧ����������˲��������������NaOH��Һ�У���ʣ1.12g���壬�ù�����һ�ֿɱ����������ĵ��ʶ������ڳ�ʪ�Ŀ������ױ���������ΪFe�������ʵ���=$\frac{1.12L}{56g/mol}$=0.02mol���������л�����Al2O3��������=2.14g-1.12g=1.02g�����ʵ���=$\frac{1.02g}{102g/mol}$=0.01mol���������Ϊ�������������ԭ���غ��֪������Fe��Oԭ����Ŀ֮��=0.02��0.01��3=2��3���ʼ�ΪFe2O3��ԭ�����ĩ�к���Al���ʣ�
���Ϸ����������������������ֵ��ʷ�Ӧ����K2S��N2��CO2�����������ԭ��Ӧ��֪���������ֵ���ΪC��S����ԭ���غ��֪�������Һ���K��N��O����Ԫ�أ���K��N��Oԭ����Ŀ֮��=0.1��2��0.1��2��0.3��2=211��3������ΪKNO3��
��1��������������֪���ҵĻ�ѧʽΪKNO3����ΪK2S������ʽΪ��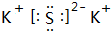 ��
��
�ʴ�Ϊ��KNO3��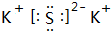 ��
��
��2����ըҩ��ը��Ӧ�Ļ�ѧ����ʽΪ��S+2KNO3+3C$\frac{\underline{\;\;��\;\;}}{\;}$K2S+N2��+3CO2����
�ʴ�Ϊ��S+2KNO3+3C$\frac{\underline{\;\;��\;\;}}{\;}$K2S+N2��+3CO2����
��3��������������Ӧ�Ļ�ѧ����ʽΪ��2Al+Fe2O3$\frac{\underline{\;����\;}}{\;}$Al2O3+2Fe���������������ǣ��ṩ��ը��Ӧ���������������
�ʴ�Ϊ��2Al+Fe2O3$\frac{\underline{\;����\;}}{\;}$Al2O3+2Fe���ṩ��ը��Ӧ���������������
��4��Al���������������ȼ����������ȷ�Ӧ���ų��������ȣ��ʿ�����CuO������������
�ʴ�Ϊ��D��
��5�����һ��ʵ�鷽����̽�����ڳ�ʪ�Ŀ����б������ļ�̬�����巽��Ϊ���ʴ�Ϊ��ȡ�����������ܽ����Һ�ֳ����ݣ�һ�ݵμ����軯����Һ����죬˵����Fe3+����һ�ݼ�K3[Fe��CN��6]�����軯�أ���Һ������ɫ����˵����Fe2+��
���� ���⿼�������ƶϣ����ڼ������ƶϣ��ۺϿ���ѧ���ļ������������������������ѶȽϴ�

 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�| A�� | $\frac{��}{400}$mol•L-1 | B�� | $\frac{20}{��}$mol•L-1 | C�� | $\frac{50��}{41}$mol•L-1 | D�� | $\frac{25��}{41}$mol•L-1 |

| A�� | ������NaOH��Һ��Ӧ��ÿmol����NaOH�����ʵ���֮��Ϊ1��1 | |
| B�� | ��������ˮ��Ӧ��ÿmol����Br2�����ʵ���֮��Ϊ3��2 | |
| C�� | ������H2�����ӳɷ�Ӧ��ÿmol����H2�����ʵ���֮��Ϊ4��7 | |
| D�� | ������O2����������Ӧ��ÿmol����O2�����ʵ���֮��Ϊ13��15 |
| A�� | D��E��ԭ�Ӹ�����2��1�γɵĻ����ֻ�����Ӽ� | |
| B�� | Ԫ��A��B��C�ĵ��ʾ����������ͬ�����͵ľ��壬Ҳ�����Dz�ͬ���͵ľ��壮���B��A2B7CD�����������ֲ�ͬ�Ļ�������÷��Ӳ��������ᷴӦ | |
| C�� | ��E��ij�����ӵ�ˮ��Һ���μ�ij�ֺ�A��C��A��C��DԪ����ɵ����ӵ���Һ��������������� | |
| D�� | B��C�γɵĻ�������е�һ������A��B�γɵĻ����� |
| A�� | һ�������£�2 mol SO2��1 mol O2������ܱ������г�ַ�Ӧ�������еķ���������2NA | |
| B�� | 256 g S8�����к�S-S��Ϊ7NA���� | |
| C�� | ��1 mol CH3COONa������CH3COOH�γɵ�������Һ�У�CH3COO-��ĿΪNA�� | |
| D�� | 1 mol Na��O2��ȫ��Ӧ������Na2O��Na2O2�Ļ���ת�Ƶ�������ΪNA�� |
��1�����������ǵ�����������أ������������ݵĹ��ά����Ҫ�ɷ��Ļ�ѧʽ��SiO2��
��2���մɡ�ˮ��Ͳ����dz��õĹ����β��ϣ����У�������ͨ��������Ҫԭ����ʯӢɰ�����ʯ��ʯ��
��3���ߴ������ִ���Ϣ���뵼��������Ȳ�ҵ����Ҫ�Ļ������ϣ���ҵ���ᴿ���ж���·�ߣ�����һ�ֹ�������ʾ��ͼ����Ҫ��Ӧ���£�
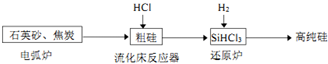
��ʯӢɰ�ͽ�̿�ڵ绡¯�и��¼���������̼��Ӧ�Ļ�ѧ����ʽΪSiO2+2C$\frac{\underline{\;����\;}}{\;}$Si���ֹ裩+2CO����
������������Ӧ�IJ����У�SiHCl3��Լռ85%������SiCl4��SiH2Cl2��SiH3Cl�ȣ�����������Ҫ������Ӧ�Ļ�ѧ��Ӧ����ʽΪ2Si���ֹ裩+6HCl=2SiHCl3+2H2���������з��ӽṹΪ������������ʵ�����Ϊ���Ȼ�������ȹ��飮
���й����ʵķе��������±���
| ���� | Si | SiCl4 | SiHCl3 | SiH2Cl2 | SiH3Cl | HCl | SiH4 |
| �е�/�� | 2355 | 57.6 | 31.8 | 8.2 | -30.4 | -84.9 | -111.9 |
| X | Y | Z | M | R | Q | |
| ԭ�Ӱ뾶/nm | 0.186 | 0.074 | 0.099 | 0.143 | ||
| ��Ҫ���ϼ� | -4��+4 | -2 | -1��+7 | +3 | ||
| ���� | �����¸õ���Ϊ��ɫ���� | ���ǽ������ϵ����� | ��ɫ��Ӧ�ʻ�ɫ | ������������ͻ���� |
��2��Y��R��ȣ��ǽ����Խ�ǿ����Cl����Ԫ�ط��ű�ʾ����������ʵ��֤����һ���۵���bc������ĸ��ţ�
a��������Y�ĵ��ʳʹ�̬��R�ĵ��ʳ���̬
b���ȶ��ԣ�HR��YH4
c��Y��R�γɵĻ�����Y������
��3�����ݱ��������Ʋ⣬Y��ԭ�Ӱ뾶����С��Χ��0.099nm��r��Si����0.143nm��
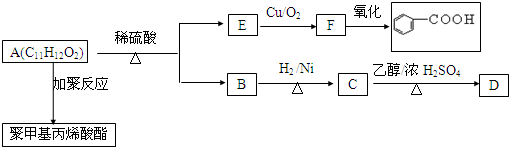
��4���ס���������ijЩԪ�ص�����������Ӧ��ˮ����Ҽ�+�ҡ���+ˮ��������ˮ��Һ�ʼ��ԣ�����Ļ�ѧʽ��NaAlO2��Na2SiO3��
��5����֪����X��ȼ����Ϊ296kJ/mo1��1mo1XM2��g��������Ϊ1mo1XM3��g���ġ�H=-99kJ/mo1��д����XM2����XM3���Ȼ�ѧ��Ӧ����ʽ2SO2��g��+O2=2SO3��g����H=-198kJ/mol��������X��s������3mo1XM3��g���ġ�H=-1185kJ/mol��
 ��
��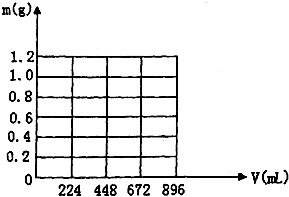 ��KOH��Ca��OH��2�����1.86gȫ������һ����ˮ���γ�ϡ��Һ���ٻ���ͨ��������CO2���壮�����ɳ����������պ����ʱ������CO2�����Ϊ224mL����״��������CO2����ˮ��������������ͬ����
��KOH��Ca��OH��2�����1.86gȫ������һ����ˮ���γ�ϡ��Һ���ٻ���ͨ��������CO2���壮�����ɳ����������պ����ʱ������CO2�����Ϊ224mL����״��������CO2����ˮ��������������ͬ����