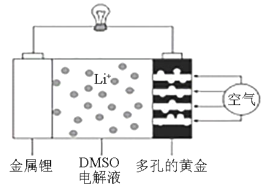
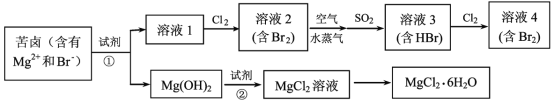
��Ŀ����
����Ŀ������ҽҩ�������ߴ��Ƚ�����������ѧ��������������������Ҫ�����á��Ӻ��⻯�ط�Һ�����ܻ�����I2��IO3-���л���I2��ʵ��������£�
��1��ȷ����Ĵ�����ʽ
��I2��ȷ����ȡ�����Һ�����Թܣ�����CCl4�����ã�����Ϊ_____��ȷ������I2��
��IO3-��ȷ����ȡ�����ϲ���Һ���������������Ƶ�0.1mo/LFeSO4��Һ������������____����ʹ֮��ַ�Ӧ���ټ�������CCl4��CCl4������ɫ���֣�˵���ú����Һ����IO3-��
��2����Ļ���
�ں����Һ�м���������K2Cr2O7��Һ��ϡH2SO4����ַ�Ӧ��ѹ���˵õ��ֵ⣬�ɴֵ��ᴿ���Ƶ��װ����ͼ��
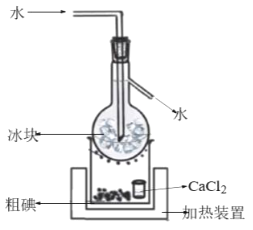
�ٲ�ȫ�õ��ֵ�����ӷ���ʽ��______��
��Cr2O72-+��I-+�� =�� +��Cr3++�� ��
��CaCl2��������__��
��3����Ĵ��ȷ�������֪��2S2O32-+I2=2I-+S4O62-��
��ȷ��ȡ0.1136g���ƺ�ĵ�����250mL����ƿ�У�����0.5gKI��30mLˮ��������ȫ�ܽ⣨KI�������ܽ⣩����0.0513mol��L-1��Na2S2O3��Һ���ٵζ�������ɫ������1mL���ҵ�����Һ�������ζ���____������Na2S2O3��Һ17.26mL����Ĵ��ȵļ������ʽΪ____��
���𰸡���Һ�ֲ㣬�ϲ������ɫ���²���ɫ ��ԭIO3-����I2 Cr2O72-+6I-+14H+=2Cr3++3I2+7H2O ��ˮ�� ���������һ��Na2S2O3��Һ������ƿ����Һ��ɫ��ʧ���Ұ�����ڲ���ɫ ![]() ��100%
��100%
��������
�Ţ�ȡ�����Һ�����Թܣ�����CCl4����ȡ�ֲ㣬CCl4���ܶ�ˮ������FeSO4��IO3����Ӧ���ɵ��ʵ�������ӡ�
�Ƣ�CaCl2����������ˮ����
�Ǽ���1mL���ҵ�����Һ�������ζ������������һ��Na2S2O3��Һ������ƿ����Һ��ɫ��ʧ���Ұ�����ڲ���ɫ�����ݷ�Ӧ����ʽ2S2O32 + I2 = 2I�� + S4O62�õ����ʵ�����ʵ������ټ����Ĵ��ȡ�
�Ţ�ȡ�����Һ�����Թܣ�����CCl4�����ã�����CCl4���ܶ�ˮ���������Ϊ��Һ�ֲ㣬�ϲ������ɫ���²���ɫ��ȷ������I2���ʴ�Ϊ����Һ�ֲ㣬�ϲ������ɫ���²���ɫ��
��ȡ�����ϲ���Һ���������������Ƶ�0.1 molL1 FeSO4��Һ������FeSO4��IO3����Ӧ���ɵ��ʵ�������ӣ�������������ǻ�ԭIO3������I2���ʴ�Ϊ����ԭIO3������I2��
�Ƣٵõ��ֵ�����ӷ���ʽ��Cr2O72 + 6 I��+14 H+= 3 I2+ 2Cr3++ 7H2O���ʴ�Ϊ��Cr2O72 + 6 I��+14 H+= 3 I2+ 2Cr3++ 7H2O��
��CaCl2����������ˮ�����ʴ�Ϊ����ˮ����
����0.0513mol��L-1��Na2S2O3��Һ���ٵζ�������ɫ������1mL���ҵ�����Һ�������ζ������������һ��Na2S2O3��Һ������ƿ����Һ��ɫ��ʧ���Ұ�����ڲ���ɫ������Na2S2O3��Һ17.26mL�����ݷ�Ӧ����ʽ2S2O32 + I2 = 2I�� + S4O62�õ����ʵ�����ʵ���n(I2) = ![]() ��0.0513mol��L-1��0.01726L =
��0.0513mol��L-1��0.01726L = ![]() ��0.0513��0.01726 mol����˵�Ĵ��ȵļ������ʽΪ
��0.0513��0.01726 mol����˵�Ĵ��ȵļ������ʽΪ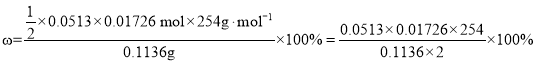 ���ʴ�Ϊ�����������һ��Na2S2O3��Һ������ƿ����Һ��ɫ��ʧ���Ұ�����ڲ���ɫ��
���ʴ�Ϊ�����������һ��Na2S2O3��Һ������ƿ����Һ��ɫ��ʧ���Ұ�����ڲ���ɫ��![]() ��
��