��Ŀ����
17��ԭ�ӽṹ��Ԫ�����ڱ�������������ϵ��������ѧԭ�ӽṹ����ش��������⣺��1������Ϊ��21���͵Ľ��������ѣ�Ti��Ԫ��ԭ�ӵļ۵����Ų�ʽΪ��3d24s2��
��2��д��3p�������2��δ�ɶԵ��ӵ�Ԫ�صķ��ţ�Si��S��
��3��д��������Ԫ����S�����������P�����������ȵ�Ԫ�ط��ţ�O��Mg��
��4��ԭ�ӵĺ˵����Ϊ33������Χ�����Ų���4s24p3������Ԫ�����ڱ��е�λ���ǵ������ڵ�VA�壮
��5���ճ������й㷺Ӧ�õIJ���֣���������������������ij��Ԫ�أ���Ԫ�صļ۵����Ų�ʽΪ3d54s1����Ԫ�ص�����Ϊ������
���� ��1���ѣ�Ti����22��Ԫ�أ�ԭ�Ӻ��������Ϊ22��
��2��3p�������2��δ�ɶԵ��ӣ���Χ�����Ų�Ϊ3s23p2��3s23p4��
��3��������Ԫ���У�ԭ�Ӻ���p�����������s�����������ȣ���������Ų�Ϊ1s22s22p4��1s22s22p63s2��
��4��ԭ�ӵĺ˵����Ϊ33����ΪAsԪ�أ���4s��4p����Ϊ����Χ���ӣ������������ԭ����д����Χ�����Ų�ʽ������Ԫ�صĵ��Ӳ���������������ȡ�����������������������ȣ�
��5����Ԫ�صļ۵����Ų�ʽΪ3d54s1��������Ų�Ϊ[Ar]3d54s1����֪ԭ������Ϊ24��
��� �⣺��1���ѣ�Ti����22��Ԫ�أ�ԭ�Ӻ��������Ϊ22����������Ų�ʽΪ1s22s22p63s23p63d24s2���۵����Ų�ʽΪ3d24s2���ʴ�Ϊ��3d24s2��
��2��3p�������2��δ�ɶԵ��ӣ���Χ�����Ų�Ϊ3s23p2��3s23p4����Ϊ��Χ�����Ų�Ϊ3s23p2����SiԪ�أ���Ϊ��Χ�����Ų�Ϊ3s23p4����SԪ�أ�
�ʴ�Ϊ��Si��S��
��3��������Ԫ���У�ԭ�Ӻ���p�����������s�����������ȣ���������Ų�Ϊ1s22s22p4��1s22s22p63s2��ԭ�������ֱ�Ϊ8��12����ΪO��Mg���ʴ�Ϊ��O��Mg��
��4��ԭ�ӵĺ˵����Ϊ33����ΪAsԪ�أ���4s��4p����Ϊ����Χ���ӣ������������ԭ��֪����Χ�����Ų�ʽ4s24p3������Ԫ�صĵ��Ӳ���������������ȡ�����������������������ȣ�����AsԪ��λ�ڵ������ڵ�VA�壬
�ʴ�Ϊ��4s24p3���������ڵ�VA�壻
��5����Ԫ�صļ۵����Ų�ʽΪ3d54s1��������Ų�Ϊ[Ar]3d54s1����֪ԭ������Ϊ24����Ԫ��ΪCr������Ϊ�����ʴ�Ϊ������
���� ���⿼��Ԫ�����ڱ��Ľṹ��Ӧ�ã�Ϊ��Ƶ���㣬����ԭ�Ӻ�������Ų���Ԫ�ص�λ�ü��ṹ�Ĺ�ϵΪ���Ĺؼ������ط�����Ӧ�������Ŀ��飬��Ŀ�ѶȲ���

 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�| A�� | CH3 COOH�ĵ���ƽ�ⳣ������Ϊԭ����10�� | |
| B�� | CH3 COOһ��CH3 COOH��Ũ��֮������Ϊԭ����10�� | |
| C�� | CH3 COOһ��Hʮ��Ũ��֮����СΪԭ����0.1�� | |
| D�� | CH3 COOH��CH3 COO-��Ũ��֮�ͼ�СΪԭ����0.1�� |
| A�� | һ������CO2 | B�� | һ������HBr�����ܺ���CO2 | ||
| C�� | һ������Cl2��CO2 | D�� | ���ܺ���CO2��Cl2 |
| A�� | ����ϩ�ɷ����ӳɷ�Ӧ | |
| B�� | ���ά��������֬�����ɸ߷��ӻ�������ɵ����� | |
| C�� | ú�Ϳ���ʯ�ͷ����ã�������ȼ�Ϻͱ������������� | |
| D�� | ��ϩ�ͱ�����ʹ��ˮ��ɫ����ɫ��ԭ����ͬ |
| A�� | �ӳɡ���ȥ��ȡ�� | B�� | ��ȥ���ӳɡ�ȡ�� | C�� | ȡ������ȥ���ӳ� | D�� | ȡ�����ӳɡ���ȥ |
| A�� | ��ѧ��Ӧ��Ȼ���������仯 | |
| B�� | ��ѧ��Ӧ�е������仯��Ҫ���ɻ�ѧ���Ķ��Ѻ��γ������ | |
| C�� | ����������ķ�Ӧ�����Է���Ӧ | |
| D�� | Ҫ�жϷ�Ӧ���еķ������ۺϿ�����ϵ���ʱ���ر� |
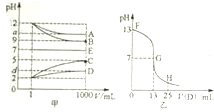 ����A��E�������±��е�������ɵģ������½������ʵ���Һ��1mLϡ�͵�1000mL��pH�ı仯��ϵ���ͼ��ʾ������A��D��Ӧ�õ�E����ش��������⣮
����A��E�������±��е�������ɵģ������½������ʵ���Һ��1mLϡ�͵�1000mL��pH�ı仯��ϵ���ͼ��ʾ������A��D��Ӧ�õ�E����ش��������⣮| ������ | NH4+��H+��Na+ |
| ������ | OH-��CH3COO-��Cl- |
��2��д��A��C��Ӧ�����ӷ���ʽ��NH3+H+=NH4+��
��3��ͼ��Ϊ����ʱ��25mLijŨ�ȵ�B��Һ����εμ�0.2mol��L-1��D��Һ�Ĺ�����pH�ı仯���ߣ�
��ͼ����B�����ʵ���Ũ��Ϊ0.1mol��L-1
��G����Һ�����ԣ������ǡ����ȫ��Ӧ�ĵ�����FG����FG��GH�����䣮
��G����Һ�и�����Ũ�ȴ�С��ϵ��c��Na+��=c��CH3COO-����c��H+��=c��OH-����
��4��t��ʱ��A��ϡ��Һ��c��H+��=10-amol��L-1��c��OH-��=10-bmol��L-1����֪a+b=13�����¶��£�t�棩����100mL0.2mol��L-1��C��Һ��100mL0.4mol��L-1��B��Һ��Ϻ���Һ����仯���Բ��ƣ�����Һ��pH=12��
| A�� | 18 gˮ�������ĵ�����Ϊ10NA | |
| B�� | �ڱ�״���£�1molˮ�����ԼΪ22.4L | |
| C�� | 0.3 mol•L-1Na2SO4��Һ�к�0.6NA��Na+ | |
| D�� | 11.2L�����к�NA����ԭ�� |