��Ŀ����
16�����ʵĽṹ�������ʣ����ʷ�ӳ��ṹ�ص㣮��1�����ʯ��ʯī��̼Ԫ�ص����ֳ������ʣ�������������ȷ����ac
a�����ʯ��̼ԭ�ӵ��ӻ�����Ϊsp3�ӻ���ʯī��̼ԭ�ӵ��ӻ�����Ϊsp2�ӻ�
b�������й��ۼ��ļ��������ʯ��C-C��ʯī��C-C
c��������۵㣺���ʯ��ʯī
d�������й��ۼ��ļ��ǣ����ʯ��ʯī
��2��ijʯ��ķ��ӽṹ��ͼ1��ʾ��
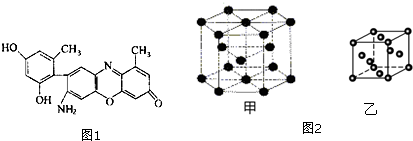
��ʯ���������Ԫ���У���һ��������С�����˳����C��H��O��N����̬ԭ��2p����������ɵ����ӵ���C��O����Ԫ�ط��ţ��� ����������Ԫ���γɵ��������͵�һ����������H3O+���ѧʽ����
�ڸ�ʯ�����ܽ���ˮ���������ܵ�ԭ����ʯ������к���-OH��-NH2��������H2O�γ�������ɽṹ֪���÷���Ϊ���Է��ӣ�������������ԭ����������ˮ��
��3��ͭ����Ͻ�����������ʹ�õĽ������ϣ�
��NF3����NH3��F2��Cu���������·�Ӧֱ�ӵõ���4NH3+3F2$\frac{\underline{\;Cu\;}}{\;}$NF3+3NH4F��������ѧ����ʽ�е�5�����������ľ���������abd������ţ���
a�����Ӿ��� b�����Ӿ��� c��ԭ�Ӿ��� d���������壬
��NF3��NH3��Ϊ�����Σ���ǰ����С�ں��ߣ�ԭ���ǵ縺�ԣ����������⣬NF3�й��õ��ӶԸ�ƫ������ų���С��NH3
�����ڱ���ͭ������Ԫ�صľ���ṹ��ͼ2�ף�����һ�������к���6����ԭ�ӣ�����ͭ��ȡ��ͼ2����ʾ�ѻ���ʽ���ɳ�Ϊ�����������ܶѻ���
���� ��1��a������̼ԭ���γɵĦҼ�����ȷ��ԭ���ӻ���ʽ��
bc��sp2�ӻ��У�s����ijɷֱ�sp3�ӻ����࣬����ʯī��̼ԭ�ӻ��д�м������γɵĹ��ۼ����̣����ι̣���ʯī�IJ��ڹ��ۼ������Ƚ��ʯ�ļ����̣������������ƻ���ѧ����Ҫ����������
d�����ʯ��̼ԭ�����ĸ�̼ԭ���γ�4�����۵����������������壬ʯī�е�̼ԭ����sp2�ӻ���������ڵ�����̼ԭ���ԦҼ���ϣ��γ��������ε�ƽ���״�ṹ��
��2����ʯ���������Ԫ��N��O��C��H������Ԫ�������ɣ�ͬ����Ԫ�ش������ҵ�һ�����������ڢ�A���Ԫ����������㴦�ڰ���״̬����һ���ȶ��ṹ�����Ե�һ�����ܸ������ڵ�ͬ��������Ԫ�أ�ʯ���������Ԫ���У���̬ԭ��2p����������ɵ����ӵ���C��OԪ�أ�����������Ԫ���γɵ��������͵�һ���������м۲���ӶԸ�����4�Һ���һ���µ��Ӷԣ�
������Ĵ��ڵ������ʵ��ܽ��������ٽ����������ԭ��������
��3���ٸ������ʵĹ�����ȷ���������ͣ�
�ڸ��ݵ縺���жϹ��ۼ�֮����ų�����С������ȷ�����ǵĴ�С��
�۸��ݼ����Ľṹͼ�����þ�̯�����㾧������ԭ���������ݽ���ͭ�����Ľṹͼ�ж϶ѻ���ʽ��
��� �⣺��1��a�����ʯ��̼ԭ�����ĸ�̼ԭ���γ�4�����۵����������������壬̼ԭ�ӵ��ӻ�����Ϊsp3�ӻ���ʯī�е�̼ԭ�������ڵ�����̼ԭ���ԦҼ���ϣ��γ�ƽ���������νṹ��̼ԭ�ӵ��ӻ�����Ϊsp2�ӻ�����a��ȷ��
b��sp2�ӻ��У�s����ijɷֱ�sp3�ӻ����࣬����ʯī��̼ԭ�ӻ��д�м������γɵĹ��ۼ����̣����ι̣���ʯī�IJ��ڹ��ۼ������Ƚ��ʯ�ļ����̣���b����
c��ʯī�IJ��ڹ��ۼ������Ƚ��ʯ�ļ����̣������������ƻ���ѧ����Ҫ�������������Ծ�����۵���ʯ��ʯī����c��ȷ��
d�����ʯ��̼ԭ�����ĸ�̼ԭ���γ�4�����۵����������������壬����Ϊ109��28�䣬ʯī�е�̼ԭ����sp2�ӻ���������ڵ�����̼ԭ���ԦҼ���ϣ��γ��������ε�ƽ���״�ṹ������Ϊ120�㣬��d����
��ѡa c��
��2����ʯ���������Ԫ��N��O��C��H������Ԫ�������ɣ�ͬ����Ԫ�ش������ҵ�һ�����������ڢ�A���Ԫ����������㴦�ڰ���״̬����һ���ȶ��ṹ�����Ե�һ�����ܸ������ڵ�ͬ��������Ԫ�أ�����N��O��C��H��һ��������С�����˳����C��H��O��N��ʯ���������Ԫ���У���̬ԭ��2p����������ɵ����ӵ���C��OԪ�أ�����������Ԫ���γɵ��������͵�һ���������м۲���ӶԸ�����4�Һ���һ���µ��Ӷԣ�����ΪH3O+��
�ʴ�Ϊ��C��H��O��N��C��O��H3O+��
��ʯ������к���-OH��-NH2��������H2O�γ����������Ĵ��ڵ������ʵ��ܽ��������ɽṹ֪���÷���Ϊ���Է��ӣ�������������ԭ����ʯ��������ˮ��
�ʴ�Ϊ��ʯ������к���-OH��-NH2��������H2O�γ�������ɽṹ֪���÷���Ϊ���Է��ӣ�������������ԭ����������ˮ��
��3����NH3��F2��NF3�Ĺ�����Ϊ���ӣ�Cu�Ĺ������ǽ��������Ӻ����ɵ��ӣ�NH4F�Ĺ��������������ӣ������⼸��������NH3��F2��NF3Ϊ���Ӿ��壬CuΪ�������壬NH4FΪ���Ӿ��壬
��ѡabd��
�����ڵ縺�ԣ����������⣬NF3�й��õ��ӶԸ�ƫ������ų���С��NH3������NF3�ļ���С��NH3�ļ��ǣ�
�ʴ�Ϊ���縺�ԣ����������⣬NF3�й��õ��ӶԸ�ƫ������ų���С��NH3��
�۸��ݼ����Ľṹͼ�����þ�̯����֪��������ԭ����Ϊ$12��\frac{1}{6}+2��\frac{1}{2}+3$=6�����ݽ���ͭ�����Ľṹͼ��֪��ͭԭ�ӷֲ��ھ���������Ķ���������ϣ����Զѻ���ʽΪ�����������ܶѻ���
�ʴ�Ϊ��6�������������ܣ�
���� ���⿼���˾����ļ��㡢�������͵��жϡ������֪ʶ�㣬�������ʵĹ�����ȷ���������ͣ�֪����������ԭ����������������ʵ�Ӱ���֪ʶ�㣬��Ŀ�ѶȲ���

| A�� | NaOH | B�� | HCl | C�� | H2SO4 | D�� | KCl |
��CO2��CO�����ʵ���Ũ��֮��Ϊ1��2��״̬
�ڻ��������ܶȲ��ٸı��״̬
�ۻ�������ѹǿ���ٸı��״̬
�ܵ�λʱ��������nmolCO2��ͬʱ����nmolC
�ݵ�λʱ��������nmolCO2 ��ͬʱ����2nmolCO
��C���������ٸı��״̬��
| A�� | �٢ܢݢ� | B�� | �ڢۢݢ� | C�� | �ڢܢݢ� | D�� | �٢ڢۢ� |
��ȡ����Ӧ���ڼӳɷ�Ӧ������ȥ��Ӧ����ʹ��ˮ��ɫ����ʹ����KMnO4��Һ��ɫ������AgNO3��Һ���ɰ�ɫ�������Ӿ۷�Ӧ��
| A�� | ���Ϸ�Ӧ���ɷ��� | B�� | ֻ�Т߲��ܷ��� | ||
| C�� | ֻ�Т��ܷ��� | D�� | ֻ�Тڲ��ܷ��� |

 ��9g������������������ȫ��Ӧ�����������ڱ�״���µ������22.4L�������������С�մ���Һ��ȫ��Ӧ�����������ڱ�״���µ������22.4L��
��9g������������������ȫ��Ӧ�����������ڱ�״���µ������22.4L�������������С�մ���Һ��ȫ��Ӧ�����������ڱ�״���µ������22.4L��