��Ŀ����
17����������AgCl�ֱ�������������У�AgCl���ܽ���ɴ�С���е�˳���ǣ���������20mL 0.01mol•L-1NH4Cl��Һ ��
��30mL 0.02mol•L-1 CaCl2��Һ
��40mL 0.03mol•L-1���ᡡ
��10mL����ˮ��
��50mL 0.05mol•L-1AgNO3��Һ��
| A�� | �٢ڢۢܢ� | B�� | �ܢ٢ۢڢ� | C�� | �ݢܢڢ٢� | D�� | �ܢۢݢڢ� |
���� AgCl������Һ�д����ܽ�ƽ�⣺AgCl��aq��?Ag+��aq��+Cl-��aq����AgCl���ܽ�ȴ�Сȡ������Һ��c��Ag+����c��Cl-���Ĵ�С��c��Ag+����c��Cl-��ԽС��AgCl���ܽ��Խ��ƽ���ƶ��ĽǶȽ��з������ɣ�
��� �⣺����c��Ag+����c��Cl-����С�Ƚϣ�c��Ag+����c��Cl-��ԽС��AgCl���ܽ��Խ��c��Cl-��=0.01mol/L����c��Cl-��=0.04mol/L����c��Cl-��=0.03mol/L����c��Ag+����c��Cl-��Ϊ0����c��Ag+��=0.05mol/L����AgCl���ܽ���ɴ�С����˳���Ǣܣ��٣��ۣ��ڣ��ݣ�
��ѡB��
���� ���⿼�����ܵ���ʵ��ܽ�ƽ�⣬��Ŀ�ѶȲ���ע���������ܵ���ʵ��ܽ�ƽ��ĺ��弰��Ӱ�����أ���ȷ��Һ�к��е������ӻ�������Ũ��Խ���Ȼ������ܽ��ԽС��

��ϰ��ϵ�д�
 ��ĩ100�ִ��غ�������ϵ�д�
��ĩ100�ִ��غ�������ϵ�д� Сѧ�������Ծ�ϵ�д�
Сѧ�������Ծ�ϵ�д�
�����Ŀ
8����NA��ʾ�����ӵ�����������˵����ȷ���ǣ�������
| A�� | 1mol�����μ�������ԭ��Ӧ��ת�Ƶĵ�����һ��Ϊ2 NA | |
| B�� | 0.1mol Fe��0.1mol Cl2��ַ�Ӧ��ת�Ƶĵ�����Ϊ0.3NA | |
| C�� | ���³�ѹ�£�44g������̼���������γɵĹ��õ��Ӷ���Ϊ2 NA | |
| D�� | ���³�ѹ�£�1.6gO2��O3�����������������Ϊ0.8NA |
12��������װ�ý�����Ӧʵ�飬�ܴﵽʵ��Ŀ���ǣ�������
| A�� |  ͼΪ�������������������������Է�ֹ��������ʴ | |
| B�� |  ͼ��ʾװ�ÿ��Լ�����������NaOH����Һ���Ȳ�������ϩ | |
| C�� |  ͼ��ʾװ�ÿ��������Ʊ����������� | |
| D�� |  ͼ��ʾװ�ÿ��������ⶨ�к��� |
2������пƬ�ʹ�ͭƬ�õ������Ӻ����ϡ�����з���ԭ��ط�Ӧ������������ȷ���ǣ�������
| A�� | ������O2�ݳ� | B�� | ����������SO42-Ũ�������� | ||
| C�� | ����ͨ��������ͭƬ����пƬ | D�� | ͭƬ���淢��������Ӧ |
9������������ϵͳ�����У���ȷ���ǣ�������
| A�� | 2��3-�������� | B�� | 2-�������� | ||
| C�� | 3��3-�������� | D�� | 3-��-2-�һ����� |
6�����ж��л�������ķ�������ȷ���ǣ�������
| A�� | ��ϩ��CH2=CH2�������� ���������飨 ���������飨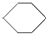 ��������֬���� ��������֬���� | |
| B�� | ����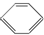 ���������飨 ���������飨 ���������飨 ���������飨 ��ͬ���ڷ����� ��ͬ���ڷ����� | |
| C�� | ��ϩ��CH2=CH2������Ȳ��CH��CH��ͬ����ϩ�� | |
| D�� |  �� �� �� �� ͬ���ڻ����� ͬ���ڻ����� |
7�������±����������ʵ�������ݣ����ش��������⣺
��1��NH3�ķе��PH3�ߵ�ԭ����NH3���Ӽ京�������PH3���Ӽ京�з��»���������ȷ��»���ǿ��
��2��CH4�ķֽ��¶ȱ�SiH4�ߵ�ԭ����C-H���ļ��ܴ���Si-H���ļ��ܣ�N-H���ļ��ܴ���P-H���ļ��ܣ���˷ֽ��¶�CH4�ķֽ��¶ȸ���SiH4��NH3�ķֽ��¶ȸ���PH3��
| CH4 | SiH4 | NH3 | PH3 | |
| �е㣨K�� | 101.7 | 161.2 | 239.7 | 185.4 |
| �ֽ��¶ȣ�K�� | 873 | 773 | 1073 | 713.2 |
��2��CH4�ķֽ��¶ȱ�SiH4�ߵ�ԭ����C-H���ļ��ܴ���Si-H���ļ��ܣ�N-H���ļ��ܴ���P-H���ļ��ܣ���˷ֽ��¶�CH4�ķֽ��¶ȸ���SiH4��NH3�ķֽ��¶ȸ���PH3��