��Ŀ����
����Ŀ��������һ�ַdz���Ҫ�Ļ���ԭ�ϣ��ڲ��������ϡ��ϳ�ϴ�Ӽ��ȹ�ҵ�����Ź㷺��Ӧ�á�
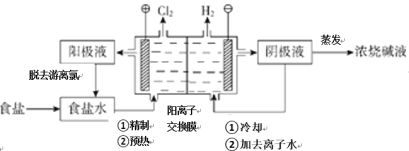
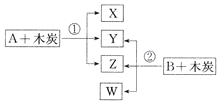
(1)��ҵ���������Ƽ����NaCl��NH3��CO2��ˮ��Ϊԭ���Ʊ�����䷴Ӧԭ��Ϊ��NaCl��NH3��CO2��H2O=NaHCO3����NH4Cl����������Ĺ�������ʾ��ͼ���£�
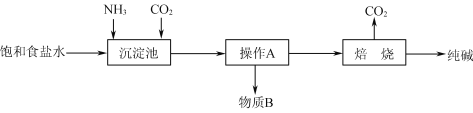
��ش��������⣺
��������NaHCO3�����п��ܺ����������������ʣ�����þ������Ƿ������������ʵIJ���������________��
�ڸù��������пɻ��������õ�������________________��
�����ƵõĴ�����ֻ��������NaCl���ⶨ�ô���Ĵ��ȣ����з�������������________(����ĸ)��
a. ��m�˴�����Ʒ�м�������CaCl2��Һ�����������ˡ�ϴ�ӡ������������Ϊb g
b. ��m�˴�����Ʒ�м�������ϡ���ᣬ�ü�ʯ��(��Ҫ�ɷ���CaO��NaOH)���ղ��������壬��ʯ������b g
c. ��m�˴�����Ʒ�м�������AgNO3��Һ�������ij��������ˡ�ϴ�ӡ������������Ϊb g
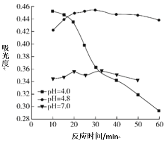
(2)��0.84 g NaHCO3��1.06 g Na2CO3��ϲ������Һ������Һ�еμ�0.10 mol��L-1ϡ���ᡣ����ͼ������ȷ��ʾ������������������CO2�����ʵ����Ĺ�ϵ����___________(����ĸ)��
A��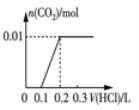 B��
B��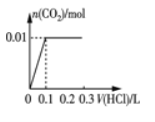
C��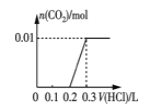 D��
D��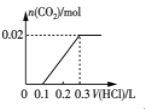
(3)����ȡ10.5 g������NaHCO3���壬����һ��ʱ���ʣ����������Ϊ8.02 g�������ʣ��Ĺ���ȫ�����뵽100 mL 2 mol��L��1�������г�ַ�Ӧ������Һ��ʣ�����������ʵ���Ũ��(����Һ������仯������Ļӷ����Բ���)___________��
���𰸡�ȡ������������ˮ����ϡ�����ữ���ټ�����������Һ����������ɫ����������Cl������֮��û�� CO2 ac D 0.75 mol��L��1
��������
(1)�����Ƽ���������ڰ������͵��Ȼ�����Һ��ͨCO2���壬��̼�����Ƶ��ܽ�ȱ�̼����С����̼�����Ƴ������ɣ������ˡ�ϴ�Ӹ�����ٽ�̼�����Ƽ��ȷֽ�ɵô��ͬʱ���ɵ�CO2����ѭ�����ã��ݴ˷������
(2)�� NaHCO3��Na2CO3��ϲ���ɵ���Һ�еμ����ᣬ�ȷ�����Ӧ��HCl+Na2CO3=NaHCO3+NaCl�����ų����壬̼���Ʒ�Ӧ��ϣ������μ�ʱ��Ȼ������Ӧ��NaHCO3+HCl=NaCl+H2O+CO2��������������̼�����ݷ���ʽ��������������������������ɶ�����̼�����ʵ������ݴ˷����жϣ�
(3)����n=![]() ����n(NaHCO3)��NaHCO3������ȫת���NaCl��������������ʵ�������NaHCO3���ʵ������ټ���ʣ��HCl���ʵ���������c=
����n(NaHCO3)��NaHCO3������ȫת���NaCl��������������ʵ�������NaHCO3���ʵ������ټ���ʣ��HCl���ʵ���������c=![]() ������Һ��ʣ�����������ʵ���Ũ�ȡ�
������Һ��ʣ�����������ʵ���Ũ�ȡ�
(1)�ټ��龧�����Ƿ��������ӣ�����ȡ������������ˮ����ϡHNO3�ữ���ٵμ�AgNO3��Һ����������ɫ��������֤���þ����к���Cl-����֮��û�У��ʴ�Ϊ��ȡ������������ˮ����ϡHNO3�ữ���ٵμ�AgNO3��Һ����������ɫ�������þ����к���Cl-����֮��û�У�
�ڱ��չ����еõ�������̼������������Ҫ������̼��������ѭ�����õ��Ƕ�����̼���ʴ�Ϊ��CO2��
��a��ֻ���Ȼ����ܺ�̼���Ʒ�Ӧ����̼��ƣ�����̼��Ƶ������Լ���̼���Ƶ��������Ը÷������У���aѡ��b��ֻ��̼�����ܺ�ϡ���ᷴӦ����CO2���ü�ʯ�����ղ��������壬��ʯ�����ص���ΪCO2��ˮ�������Լ����ܻӷ�����HCl�������������ܼ����̼�������������Ը÷��������У���b��ѡ��c��̼������Ӻ�Cl-���������ӷ�Ӧ���ɰ�ɫ���������ݳ�������������Ʒ���������Լ���̼�������������Ը÷������У���cѡ���ʴ�Ϊ��ac��
(2)0.84gNaHCO3�����ʵ���Ϊ![]() =0.01mol��1.06g Na2CO3�����ʵ���Ϊ0.01mol���� NaHCO3��Na2CO3�����Һ�еμ����ᣬ����0.01mol Na2CO3������Ӧ��HCl+Na2CO3=NaHCO3+NaCl�����ų����壬����0.1molL-1����0.1L����ʱ��Һ��̼�����Ƶ����ʵ���Ϊ0.02mol��Ȼ������Ӧ��NaHCO3+HCl=NaCl+H2O+CO2��������������̼��0.02mol̼��������ȫ��Ӧ����0.1molL-1����0.2L�����μ�������0.3Lʱ����������̼�ﵽ���ֵΪ0.02mol����ѡD��
=0.01mol��1.06g Na2CO3�����ʵ���Ϊ0.01mol���� NaHCO3��Na2CO3�����Һ�еμ����ᣬ����0.01mol Na2CO3������Ӧ��HCl+Na2CO3=NaHCO3+NaCl�����ų����壬����0.1molL-1����0.1L����ʱ��Һ��̼�����Ƶ����ʵ���Ϊ0.02mol��Ȼ������Ӧ��NaHCO3+HCl=NaCl+H2O+CO2��������������̼��0.02mol̼��������ȫ��Ӧ����0.1molL-1����0.2L�����μ�������0.3Lʱ����������̼�ﵽ���ֵΪ0.02mol����ѡD��
(3)n(NaHCO3)=![]() =0.125 mol��NaHCO3������ȫת���NaCl��������������ʵ�������NaHCO3���ʵ�������n(HCl)ʣ��=n(HCl)-n(NaHCO3)=0.1L��2molL-1-0.125 mol=0.075 mol����c(HCl)ʣ��=
=0.125 mol��NaHCO3������ȫת���NaCl��������������ʵ�������NaHCO3���ʵ�������n(HCl)ʣ��=n(HCl)-n(NaHCO3)=0.1L��2molL-1-0.125 mol=0.075 mol����c(HCl)ʣ��=![]() =0.75 molL-1���ʴ�Ϊ��0.75 molL-1��
=0.75 molL-1���ʴ�Ϊ��0.75 molL-1��

 ������ҵ����ν�����������ϵ�д�
������ҵ����ν�����������ϵ�д�����Ŀ��̽����Ƭ��Na2CO3��Һ�ķ�Ӧ��
| | |
���������� | ��Ƭ�������ϸС���� | ���ְ�ɫ���ǣ������������ݣ�������ΪH2��CO2�� |
����˵������ȷ����
A. Na2CO3��Һ�д���ˮ��ƽ�⣺CO32- + H2O HCO3- + OH-
B. �ԱȢ�˵��Na2CO3��Һ���ƻ�������ı���Ĥ
C. �Ʋ���ְ�ɫ���ǵ�ԭ��AlO2- + HCO3- + H2O = Al(OH)3��+ CO32-
D. ���Ⱥ�H2�ݳ���CO32- ˮ��ƽ���ƶ������Ӱ�����෴��