��Ŀ����
( 13��)�������������������壬�Ǿ�����������ֲ���������ڼ���ʵ��֤������ʹ�ö�����������֪������������±���ʾ��
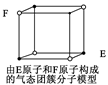
��1����Ԫ�ػ�̬ԭ�Ӻ�����ӵ�δ�ɶԵ�����Ϊ ��
��2����������ľ�������Ϊ_______ �������ڶ�����Ԫ�ص�һ�����ܴӴ�С��˳��Ϊ________________��
��3���������徧���У���ԭ�ӵ��ӻ��������Ϊ______ _��
��4���������徧���У������������������________ _��
A�����Ӽ�B��������C�����ۼ�D����λ��E�����
��5�����������۵�ͣ�������ˮ��ԭ��ֱ�Ϊ_________________________________
________________________________________________________________________��
��6��������֪������2-��-4-��������������ᱽ����Ӧ�������������塣
 �����ᱽ�� 2-��-4-������� ��������
�����ᱽ�� 2-��-4-������� ��������
��Ӧ�����У�ÿ����1mol��������,����_______���Ҽ�������_______���м�
| ����ʽ | �ṹ��ʽ | ��� | �۵� | �ܽ��� |
| C12H10ClN3O | 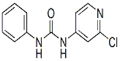 | ��ɫ�ᾧ��ĩ | 170~172��C | ������ˮ |
��2����������ľ�������Ϊ_______ �������ڶ�����Ԫ�ص�һ�����ܴӴ�С��˳��Ϊ________________��
��3���������徧���У���ԭ�ӵ��ӻ��������Ϊ______ _��
��4���������徧���У������������������________ _��
A�����Ӽ�B��������C�����ۼ�D����λ��E�����
��5�����������۵�ͣ�������ˮ��ԭ��ֱ�Ϊ_________________________________
________________________________________________________________________��
��6��������֪������2-��-4-��������������ᱽ����Ӧ�������������塣
 �����ᱽ�� 2-��-4-������� ��������
�����ᱽ�� 2-��-4-������� ����������Ӧ�����У�ÿ����1mol��������,����_______���Ҽ�������_______���м�
��13�֣���1��1 ��1�֣� ��2�����Ӿ��� N> O> C ����1�֣���2�֣�
��3��sp2��sp3 ����1�֣���2�֣� ��4��C��D ����1�֣���2�֣�
��5�����������Ƿ��Ӿ��壬���۵�ͣ�����������ˮ���Ӽ��γ��������������ˮ������1�֣���2�֣�
��6��NA����6.02��1023�� NA����6.02��1023������1�֣���2�֣�
��3��sp2��sp3 ����1�֣���2�֣� ��4��C��D ����1�֣���2�֣�
��5�����������Ƿ��Ӿ��壬���۵�ͣ�����������ˮ���Ӽ��γ��������������ˮ������1�֣���2�֣�
��6��NA����6.02��1023�� NA����6.02��1023������1�֣���2�֣�
�����������1�����ݹ���ԭ����֪����Ԫ�ػ�̬ԭ�Ӻ�����ӵ��Ų�ʽ��1s22s22p63s23p5������δ�ɶԵ�����Ϊ1����
��2������������۵�ϵͣ�������ˮ�������侧�������Ƿ��Ӿ��塣���������ڶ�����Ԫ�طֱ���C��N��O���ǽ�����Խǿ����һ������Խ�����ڵ�Ԫ�ص�2p������Ӵ��ڰ����״̬���ȶ���ǿ�����Ե�һ�����ܴ�����Ԫ�صģ�����ȷ��˳����N��O��C��
��3�����ݽṹ��ʽ��֪��2����ԭ��ȫ���γɵ���������sp3�ӻ�������һ����ԭ���γ�˫����������sp2�ӻ���
��4���������������ȫ���Ƿǽ���Ԫ�ع��ɵģ����й��ۼ������ԭ�Ӻ���ԭ�Ӷ����й¶Ե��ӣ����Ի����γ���λ������ѡCD��
��5���������������Ƿ��Ӿ��壬�����۵�ͣ������������廹����ˮ���Ӽ��γ���������Ի�������ˮ��
��6�����ڵ������ǦҼ�����˫������1���Ҽ���1���м����ɵģ����Ը��ݷ�Ӧ�ķ���ʽ��֪��ÿ����1mol��������,����NA���Ҽ���NA���м���
���������ж��ӻ��������ʱ��Ӧ��ͨ����ѧ�����У���sp3�ӻ�ȫ���γɵ�����sp2�ӻ��л���1��˫������sp�ӻ��л���1��������

��ϰ��ϵ�д�
�����Ŀ
 ����
����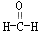 �ȣ����з�����Cԭ��ΪSP2�ӻ���
�ȣ����з�����Cԭ��ΪSP2�ӻ���