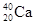��Ŀ����
��10�֣��±���Ԫ�����ڱ���һ���֣��ش������й����⣺
�ţ�����ЩԪ�ص�����������Ӧˮ�����У�������ǿ���ǣ��ѧʽ,��ͬ�� ��������ǿ���� �������Ե����������� ��
�ƣ�д����ҵ��ұ���ݵĻ�ѧ����ʽΪ ������һ�ֺ�ɫ���巴Ӧұ�����Ļ�ѧ����ʽΪ�� ��
�ǣ��ڢ�����У��ǽ����Խ�ǿ��Ԫ���� ��д��������֤�ý��۵�һ�����ӷ�Ӧ����ʽ ��
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 | ||||
| 2 | | | | | �� | | �� | | ||||
| 3 | �� | �� | �� | �� | | �� | �� | �� | ||||
| 4 | �� | 
| | | | |
 | |
�ƣ�д����ҵ��ұ���ݵĻ�ѧ����ʽΪ ������һ�ֺ�ɫ���巴Ӧұ�����Ļ�ѧ����ʽΪ�� ��
�ǣ��ڢ�����У��ǽ����Խ�ǿ��Ԫ���� ��д��������֤�ý��۵�һ�����ӷ�Ӧ����ʽ ��
�� HClO4��KOH��Al(OH)3����1�֣�
�ƣ�2Al2O3�����ڣ�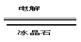 4Al��3O2����2�֣�
4Al��3O2����2�֣�
3Fe3O4��8Al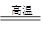 9Fe��4Al2O3��2�֣�
9Fe��4Al2O3��2�֣�
�ǣ�Cl����1�֣�Cl2��S2��=S����2Cl����2�֣�
�ƣ�2Al2O3�����ڣ�
 4Al��3O2����2�֣�
4Al��3O2����2�֣�3Fe3O4��8Al
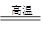 9Fe��4Al2O3��2�֣�
9Fe��4Al2O3��2�֣��ǣ�Cl����1�֣�Cl2��S2��=S����2Cl����2�֣�
��1��������ǿ��Ӧ�����Ͻǣ���F�����ۣ���ΪHClO4��������ǿ��Ӧ�����½ǣ���ΪKOH��
��3��ͬ���ڣ������ң�Ԫ�صķǽ���������ǿ
��3��ͬ���ڣ������ң�Ԫ�صķǽ���������ǿ

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ

 ��B��ͬ���ڳ�ϡ��������뾶����Ԫ�أ�C��������������ɵ����ӣ�E��F����Χ�����Ų�ʽ�ֱ�Ϊ3d54s1��3d64s2���ش��������⣺
��B��ͬ���ڳ�ϡ��������뾶����Ԫ�أ�C��������������ɵ����ӣ�E��F����Χ�����Ų�ʽ�ֱ�Ϊ3d54s1��3d64s2���ش��������⣺
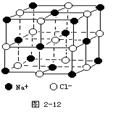
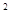 2s
2s 3s
3s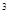 4s
4s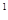 �����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ ��������������Ӧ��ˮ����Ļ�ѧʽ��
�����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ ��������������Ӧ��ˮ����Ļ�ѧʽ�� 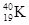 ��
��