��Ŀ����
����Ŀ�����к͵ζ����ⶨij�ռ�Ĵ��ȡ���2.5g������������(�������ᷴӦ)�Ĺ����ռ���Ʒ���Ƴ�250mL��Һ������ʵ��ش��������⣺
I.�ζ�ǰ����
��©��������ˮ��ϴ���ô�װ��Һ��ϴ��װҺ����������Һ������0���̶Ȼ���0���̶���������¼��ʼ����
II.�ζ�
�ֱ�ȡ20.00mL����Һ��3���ྻ����ƿ�У��μ�2�η�̪��Һ��Ȼ����0.2000mol��L-1�����Һ���еζ����յ㣬��¼���ն������������£�
�ζ���� | ����Һ���(mL) | �ζ�ǰ(mL) | �ζ���(mL) |
1 | 20.00 | 0.50 | 20.70 |
2 | 20.00 | 6.00 | 26.00 |
3 | 20.00 | 5.00 | 25.10 |
III.ʵ�����ݴ���
(1)������I�еIJ�������������
��___________
(2)��ͼ��ʾΪ___________ (������ʽ��������ʽ��)�ζ��ܵ�һ���֣����õζ���©ˮ��ת�������ɲ�ȡ�Ĵ�ʩ��___________
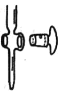
(3)�ζ��յ��������_______
(4)���㣺NaOH��Һ��Ũ��Ϊ_______mol��L-1�ռ���Ʒ�Ĵ���Ϊ________
(5)���ж����¼���������ռ�Ȳⶨ�����Ӱ��(����ƫ��������ƫС��������Ӱ����)
����������ˮ��ϴ��ƿ��ʹ�ⶨ���_____________
�����ζ�ǰ�ζ��ܼ��������ݣ��ζ���������ʧ��ʹ�ⶨ���_____________
�����ռ���ָʾ���ֲ�����ɫ�б仯��ֹͣ�ζ���ʹ�ⶨ���_____________
�ܶ���ʱ�����ζ�ǰ���ӣ��ζ����ӣ�ʹ�ⶨ���_____________
���𰸡������� ��ʽ Ϳ�Ϸ�ʿ�֣�����ת���� ���������һ�����ᣬ��Һ�ɺ�ɫ��Ϊ��ɫ���Ұ�����ڲ���Ϊԭ������ɫ 0.2010 80.4% ��Ӱ�� ƫ�� ƫС ƫС
��������
(1)������ǰ������ó��ò�Ϊ�����ݡ�
(2)�л�����Ϊ��ʽ�ζ��ܣ����õζ���©ˮ��ת�������ɲ�ȡ�Ĵ�ʩ��Ϳ�Ϸ�ʿ�֡�
(3)�õζ�����ζ����˵ζ��յ����������Һ�ɺ�ɫ��Ϊ��ɫ��
(4)��������ζ�����ʵ�����ݵó�ƽ��ֵΪ20.10mL������NaOH��Һ��Ũ�ȣ������ռ���Ʒ�Ĵ��ȡ�
(5)����������ˮ��ϴ��ƿ������Һ���ʵ���δ�䣻�����ζ�ǰ�ζ��ܼ��������ݣ��ζ���������ʧ������ƫ�ߣ������ռ���ָʾ���ֲ�����ɫ�б仯��ֹͣ�ζ�����δ�ﵽ�ζ��յ㣬�����������ƫС��������ʱ�����ζ�ǰ���ӣ��ζ����ӣ�����ƫС��
(1)������ǰ������ó��ò�Ϊ�����ݣ��ʴ�Ϊ�������ݡ�
(2)��ͼ��ʾ���л�����Ϊ��ʽ�ζ��ܵ�һ���֣����õζ���©ˮ��ת�������ɲ�ȡ�Ĵ�ʩ��Ϳ�Ϸ�ʿ�֣�����ת�������ʴ�Ϊ����ʽ��Ϳ�Ϸ�ʿ�֣�����ת������
(3)�õζ�����ζ����˵ζ��յ�������ǵ��������һ�����ᣬ��Һ�ɺ�ɫ��Ϊ��ɫ���Ұ�����ڲ���Ϊԭ������ɫ���ʴ�Ϊ�����������һ�����ᣬ��Һ�ɺ�ɫ��Ϊ��ɫ���Ұ�����ڲ���Ϊԭ������ɫ��
(4)��������ζ�����ʵ�����ݵó�ƽ��ֵΪ20.10mL��NaOH��Һ��Ũ��Ϊ![]() ���ռ���Ʒ�Ĵ���Ϊ
���ռ���Ʒ�Ĵ���Ϊ![]() ���ʴ�Ϊ��0.2010��80.4%��
���ʴ�Ϊ��0.2010��80.4%��
(5)����������ˮ��ϴ��ƿ������Һ���ʵ���δ�䣬��˲ⶨ�����Ӱ�죻�ʴ𰸣���Ӱ�졣
�����ζ�ǰ�ζ��ܼ��������ݣ��ζ���������ʧ���������ƫ�ߣ��ⶨ���ƫ�ʴ�Ϊ��ƫ��
�����ռ���ָʾ���ֲ�����ɫ�б仯��ֹͣ�ζ�����δ�ﵽ�ζ��յ㣬�������ƫС���ⶨ���ƫС���ʴ�Ϊ��ƫС��
������ʱ�����ζ�ǰ���ӣ��ζ����ӣ�����ƫС���ⶨ���ƫС���ʴ�Ϊ��ƫС��