��Ŀ����
�ظ�����ǹ�ҵ������ʵ���ҵ���Ҫ����������ҵ�ϳ��ø�������Ҫ�ɷ�ΪFeO��Cr2O3���Լ�SiO2��Al2O3�����ʣ�Ϊԭ��������ʵ����ģ�ҵ���ø�������K2Cr2O7����Ҫ�������£�
��Ӧ������Ҫ�����ķ�ӦΪ��
��.FeO��Cr2O3��NaOH��KClO3��Na2CrO4��Fe2O3��H2O+KCl��δ��ƽ��
��.Na2CO3+SiO2�� Na2SiO3+CO2��
��.Al2O3+2NaOH�� 2NaAlO2+H2O
�ڲ�����н���ҺpH���ڵ�7~8���Խ�SiO32-��AlO2-ת��Ϊ��Ӧ�ij�����ȥ��
��1���ڷ�Ӧ������������________������245g KClO3�μӷ�Ӧ����ת�Ƶĵ�����Ϊ_____________��
��2����Ӧ�������ɵ�Fe2O3�ֿɺ�Na2CO3��Ӧ�õ�һ��Ħ������Ϊ111g/mol�Ļ������ǿ��ˮ�⣬�ڲ��������ɳ�������ȥ��д�����ɸû�����Ļ�ѧ��Ӧ����ʽ_____________________________
___________________________��
��3��������Ŀ���ǽ�CrO42-ת��ΪCr2O72-��������Ϊ__________________________�����ӷ���ʽΪ_______________________________________��
��4����ѡ�ú��ʵķ�����һ���ᴿ�ֲ�Ʒ�ظ����__________������ĸ��
A���ؽᾧ B����ȡ��Һ C������
��5��������Ʒ��K2Cr2O7�Ĵ��������������ữ��K2Cr2O7��KI������I2,Ȼ������������ʲ��I2�����Ӷ����K2Cr2O7������д���ữ��K2Cr2O7��KI��Ӧ�Ļ�ѧ����ʽ________________________��
��1��KClO3��2�֣��� 12��6.02��1023��12NA��2�֣�
��2��Fe2O3��Na2CO3 2NaFeO2+CO2����2�֣�
2NaFeO2+CO2����2�֣�
��3����Һ�ɻ�ɫ��Ϊ��ɫ��2�֣� 2CrO42-��2H+ Cr2O72-��H2O ��2�֣�
Cr2O72-��H2O ��2�֣�
��4��A ��2�֣�
��5��K2Cr2O7+7H2SO4+6KI=Cr2 (SO4)3+3I2+7H2O+4K2SO4��2�֣�
���������������1���������Ƿ�Ӧ�л��ϼ۽��͵����ʣ��ڷ�Ӧ����KClO3�е���Ԫ�ػ��ϼ۴�+5����-1��Ϊ��������245g KClO3�����ʵ���Ϊ2mol����ת�Ƶ��ӵ����ʵ���Ϊ12mol����12 NA
��2��Fe2O3��Na2CO3��Ӧ��������Ӧ��CO2����һ�ֲ����к����ơ���Ԫ�أ����Ħ������Ϊ111g/mol���Ƴ��û�����Ļ�ѧʽΪNaFeO2���������ɸû�����Ļ�ѧ��Ӧ����ʽFe2O3��Na2CO3 2NaFeO2+CO2��
2NaFeO2+CO2��
��3��CrO42-��Cr2O72-����ɫ�ֱ�Ϊ��ɫ����ɫ����������Ϊ��Һ�ɻ�ɫ��Ϊ��ɫ����Ӧ�����ӷ���ʽΪ 2CrO42-��2H+
 Cr2O72-��H2O
Cr2O72-��H2O
��4����ȡ��Һ�����ڻ������ܵ�Һ�壬���������ڷе����ϴ�Ļ���Һ�壬���ؽᾧ�����������ʲ�ͬ�¶����ܽ�ȵIJ�ͬ�������ᴿ���ؽᾧ���ʵĹ��̣�����ѡ��A��
��5�������ữ��K2Cr2O7��KI��Ӧ������������ԭ��Ӧ���ۣ��û�ѧ��Ӧ����ʽK2Cr2O7+7H2SO4+6KI=Cr2 (SO4)3+3I2+7H2O+4K2SO4
���㣺�������������жϡ�����ת����Ŀ�ļ��㡢��ѧ����ʽ���ƶϡ����ӷ���ʽ����д�Լ������ᴿ����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���15�֣��ں��췢��ʱ���£�N2H4�����������ﳣ��������ƽ�����
��Һ̬�������ȼ��ʱ����Һ̬N2O4��Ϸ���������ԭ��Ӧ����֪ÿ1g�³�ַ�Ӧ��������̬ˮ�ų�����Ϊa KJ����д���÷�Ӧ���Ȼ�ѧ����ʽ ��
��ʵ������N2H4��H2O��NaOH����һ�������ռ�114��116�����ּ�Ϊ��ˮ�¡�
������������в���Ҫ�������� ���������ĸ����
| A���ƾ��� | B����ֱ�������� | C����ƿ | D��ֱ�������� |
�ڳ���������������⣬��ȱ�ٵ���Ҫ���������� ��
������ʹ��¯�ڱڵ������ɽ�Ϊ���ܵĴ�����������Fe3O4���㣬�Լ�����¯��ʴ������Ӧ��������ת��Ϊ��������ÿ����1molFe3O4����Ҫ�����µ�����Ϊ g��
�ȴ�����������Fe3O4������ɿ�д��FeO��Fe2O3��ij��ѧʵ��С��ͨ��ʵ����̽��һ��ɫ��ĩ�Ƿ���Fe3O4��CuO��ɣ�������������ɫ���ʣ���̽���������£�
������裺����1. ��ɫ��ĩ��CuO������2. ��ɫ��ĩ��Fe3O4��
����3. ��
̽��ʵ�飺
ȡ������ĩ��������ϡ�����У���������Һ�еμ�KSCN�Լ���
��������1��������ʵ�������� ��
����������Һ��Ѫ��ɫ������� ������
��Ϊ��һ��̽����������������Һ�����������ۣ������� ���������3������
����һС��ͬѧ��������������CuO�������٣����ܼ������ۺ�ʵ���������ԡ��������ϣ�Cu2+��������ˮ��Ӧ��������ɫ��Һ��Cu2+��4NH3��H2O��Cu��NH3��42+��4H2O��
��Ϊ̽���Ǽ���2���Ǽ���3��������ȡ������ĩ��ϡ�������ܽ���ټ���������ˮ��������2����������� ���������� ���������3������
��.��һ���Ϊ10 L�������У�ͨ��һ������CO��H2O����850��ʱ�������·�Ӧ��CO(g)��H2O(g) CO2(g)��H2(g)����H<0��CO��H2OŨ�ȱ仯��ͼ��ʾ��
CO2(g)��H2(g)����H<0��CO��H2OŨ�ȱ仯��ͼ��ʾ��
(1)0��4 min��ƽ����Ӧ����v(CO)��________mol/(L��min)����Ӧ�ڵ�5 minʱ��ƽ�ⳣ��K��________��
t��ʱ����Ũ��(mol/L)�ı仯
| ʱ��(min) | CO | H2O | CO2 | H2 |
| 0 | 0. 200 | 0. 300 | 0 | 0 |
| 2 | 0. 138 | 0.238 | 0.062 | 0.062 |
| 3 | c1 | c2 | c3 | c3 |
| 4 | c1 | c2 | c3 | c3 |
| 5 | 0. 116 | 0. 216 | 0. 084 | |
| 6 | 0. 096 | 0. 266 | 0. 104 | |
(2)t��(����850��)ʱ������ͬ�����з���������Ӧ�������ڸ����ʵ�Ũ�ȱ仯�����ʾ��
�ٱ���3��4 min֮�䷴Ӧ����________״̬��c1��ֵ________(�>����������<��)0.08 mol/L��
�ڷ�Ӧ��4��5 min�䣬ƽ�����淴Ӧ�����ƶ������ܵ�ԭ����________(���ţ���ͬ)������5��6 min֮����ֵ�����仯�����ܵ�ԭ����________��
A������ˮ����B�������¶�C��ʹ�ô���D����������Ũ��
��.���ȵ�ϡ�������ܽ���11.4 g�����������壬������50 mL 0.5 mol/L��KNO3��Һ��ʹ���е�Fe2��ȫ��ת����Fe3����KNO3Ҳ��ȫ��Ӧ���ų�NxOy���塣
(3)�����x��________��y��________��
(4)д���÷�Ӧ�Ļ�ѧ����ʽ��________________(x��y�þ�����ֵ��ʾ)��
(5)��Ӧ������������________��
 R ��R+H2O��NaOH+Y ��Q+NaOH��V+W+H2O ��X
R ��R+H2O��NaOH+Y ��Q+NaOH��V+W+H2O ��X W+Y
W+Y Q+Z+H2O������M��Q��Y�ǵ��ʣ�Q��Y�ڳ��¡���ѹ��Ϊ���壬Z�������Σ��ơ��ػ������������ơ�
Q+Z+H2O������M��Q��Y�ǵ��ʣ�Q��Y�ڳ��¡���ѹ��Ϊ���壬Z�������Σ��ơ��ػ������������ơ� 2CO2(g) +N2(g)�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯���ߣ�����ͼ��ʾ��
2CO2(g) +N2(g)�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯���ߣ�����ͼ��ʾ��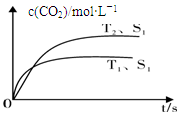
 Fe2��(aq)��S2��(aq)��Ksp=c(Fe2��)��c(S2��),������Ksp=1.0��10��16����֪FeS������Һ��c(H��)��c(S2��)֮���������������ϵ��[c(H��)]2��c(S2��)��1.0��10��22��Ϊ��ʹ��Һ��c(Fe2��)�ﵽ1 mol/L���ֽ�����FeSͶ���䱥����Һ�У�Ӧ������Һ�е�pHΪ ��
Fe2��(aq)��S2��(aq)��Ksp=c(Fe2��)��c(S2��),������Ksp=1.0��10��16����֪FeS������Һ��c(H��)��c(S2��)֮���������������ϵ��[c(H��)]2��c(S2��)��1.0��10��22��Ϊ��ʹ��Һ��c(Fe2��)�ﵽ1 mol/L���ֽ�����FeSͶ���䱥����Һ�У�Ӧ������Һ�е�pHΪ ��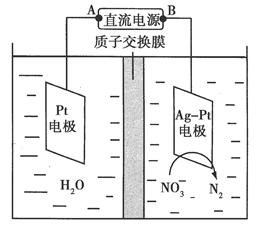

 2N2(g)��3H2O(g)����H < 0
2N2(g)��3H2O(g)����H < 0 NO2
NO2 N2
N2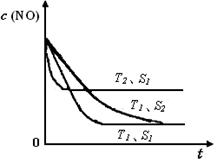
 ______ �� ______ �� N2O�� �� H2O
______ �� ______ �� N2O�� �� H2O Cu(NO3)2
Cu(NO3)2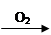 CuO
CuO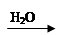 Cu(OH)2
Cu(OH)2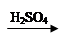 CuSO4
CuSO4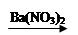 Cu(NO3)2
Cu(NO3)2