��Ŀ����
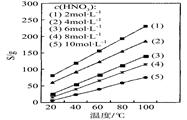
��M��R��Q��V��W��X��Y��Z�������ʣ�����֮��������¹�ϵ��
��M+Y R ��R+H2O��NaOH+Y ��Q+NaOH��V+W+H2O ��X
R ��R+H2O��NaOH+Y ��Q+NaOH��V+W+H2O ��X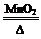 W+Y
W+Y
��X+W+H2SO4 Q+Z+H2O������M��Q��Y�ǵ��ʣ�Q��Y�ڳ��¡���ѹ��Ϊ���壬Z�������Σ��ơ��ػ������������ơ�
Q+Z+H2O������M��Q��Y�ǵ��ʣ�Q��Y�ڳ��¡���ѹ��Ϊ���壬Z�������Σ��ơ��ػ������������ơ�
�ش��������⣺
��1��д�������ŵĻ�ѧʽ�������ʽ�� M �� R ��Q ��V ��W ��X ��Y ��Z ��
��2��д�������йػ�ѧ��Ӧ�ķ���ʽ�����ӷ���ʽ�����������ת�Ʒ��������
��Ӧ�� �� ��
��Ӧ�� �� ��
��1��Na��Na2O2��Cl2��NaClO��NaCl��NaClO3��O2��Na2SO4
��2���� 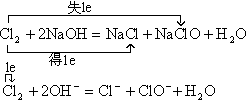
��
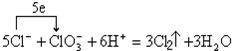
�������������R+H2O��NaOH+Y����R���ƻ�������ƣ�����R���ǵ��ʣ�����R�ǹ������ƣ�Y��������M+Y R��֪ʶM���ơ�X
R��֪ʶM���ơ�X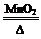 W+Y����ΪX������ػ�˫��ˮ������Wһ������ˮ������X�������ƣ���W���Ȼ��ơ�����Q+NaOH��V+W+H2O��X+W+H2SO4
W+Y����ΪX������ػ�˫��ˮ������Wһ������ˮ������X�������ƣ���W���Ȼ��ơ�����Q+NaOH��V+W+H2O��X+W+H2SO4 Q+Z+H2O�Լ�Q�����嵥�ʿ�֪��QӦ������������V�Ǵ������ƣ�Z�������ơ�
Q+Z+H2O�Լ�Q�����嵥�ʿ�֪��QӦ������������V�Ǵ������ƣ�Z�������ơ�
��1�������Ϸ�����֪M��R��Q��V��W��X��Y��Z�ֱ���Na��Na2O2��Cl2��NaClO��NaCl��NaClO3��O2��Na2SO4��
��2���������������Ʒ�Ӧ�����Ȼ��ơ��������ƺ�ˮ��Ҳ��������ԭ��Ӧ����ɱ�ʾΪ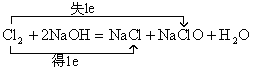 ����Ӧ���ӷ���ʽ�ɱ�ʾΪ
����Ӧ���ӷ���ʽ�ɱ�ʾΪ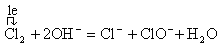 �������������������ƿ��������Ȼ��������������ɱ�ʾΪ
�������������������ƿ��������Ȼ��������������ɱ�ʾΪ ����Ӧ�����ӷ���ʽ�ɱ�ʾΪ
����Ӧ�����ӷ���ʽ�ɱ�ʾΪ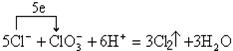 ��
��
���㣺���������ƶϵ��й��ж�

 ��У����ϵ�д�
��У����ϵ�д����������ʡ���������ƵƵ������Ϊ��ֹ�ڴ���֮���߲����У�������Ҫ�����ĸ�����������Ư���ȡ�
(1)����������Ŀǰ�����Ϲ��ϵĵ��Ĵ���Ч�����Ĺ�����������������KClO3��SO2��H2SO4�����·�Ӧ�Ƶá���д����Ӧ�����ӷ���ʽ�� ��
(2)��̼������һ������̬Ư������ѧʽ�ɱ�ʾΪNa2CO3��3H2O2��������Na2CO3��H2O2��˫�����ʡ���̼�������������ʾ��ᷢ����ѧ��Ӧ��ʧЧ�����й�̼����ֻ������������Ӧ���� ��
| A��MnO2 | B��KMnO4��Һ | C��ϡ���� | D��Na2SO3��Һ |
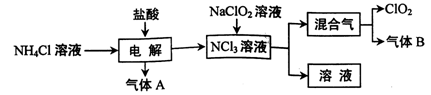
 Cu��____CuCl2��N2����____H2O��
Cu��____CuCl2��N2����____H2O��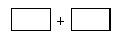


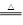 CeO2��8OH + 8_____����CeO2��8OH
CeO2��8OH + 8_____����CeO2��8OH