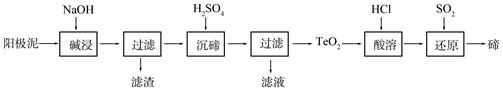
��Ŀ����
���������Ǵ�����Ⱦ��֮һ��������������ķ����ж��֡�
��1�����ü������ԭ���������֪��
CH4 (g)��4NO2(g)��4NO(g)��CO2(g)��2H2O(g) ��H ����574 kJ/mol
CH4(g)��4NO(g) �� 2N2(g)��CO2(g)��2H2O(g) ��H ����1160 kJ/mol
��CH4 ��NO2 ��ԭΪN2 ���Ȼ�ѧ����ʽΪ ��
��2������NH3����ԭ�������SCR����)���ü�����ĿǰӦ����㷺���������������ѳ������� ��Ӧ�Ļ�ѧ����ʽΪ��2NH3(g)��NO(g)��NO2(g)  2N2(g)��3H2O(g)����H < 0
2N2(g)��3H2O(g)����H < 0
Ϊ��ߵ��������ת���ʿɲ�ȡ�Ĵ�ʩ�� ��д��1�����ɣ���
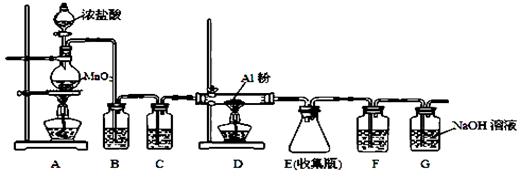
��3������ClO2�������������ת���������£�
NO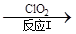 NO2
NO2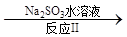 N2
N2
��֪��Ӧ��Ļ�ѧ����ʽΪ2NO+ ClO2 + H2O �� NO2 + HNO3 + HCl����Ӧ��Ļ�ѧ����ʽ�� ��������11.2 L N2����״������������ClO2 g ��
��4������CO����ԭ��������Ҳ���Դﵽ������Ⱦ��Ŀ�ġ�
��֪����һ��ʱ�������������ı���������ѧ��Ӧ���ʡ���ͼ�Ƿ�Ӧ2NO(g) + 2CO(g) 2CO2(g)+ N2(g) ��NO��Ũ�����¶�(T)�����������������(S)��ʱ��(t)�ı仯���ߡ��ݴ��жϸ÷�Ӧ�ġ�H 0 (�����������������ȷ����)�����������S1 S2 (�����������������ȷ����)��
2CO2(g)+ N2(g) ��NO��Ũ�����¶�(T)�����������������(S)��ʱ��(t)�ı仯���ߡ��ݴ��жϸ÷�Ӧ�ġ�H 0 (�����������������ȷ����)�����������S1 S2 (�����������������ȷ����)��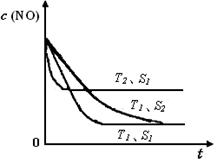
��1��CH4 (g)��2NO2(g)��N2(g)��CO2(g)��2H2O(g) ��H ����867 kJ/mol ��3�֣�
��2������NH3��Ũ�Ȼ��С��Ӧ��ϵ��ѹǿ�ͷ�Ӧ��ϵ���¶ȵȣ�������Ҳ�Ʒ֣�
��3��2NO2 + 4 Na2SO3 = N2 + 4 Na2SO4 67.5
��4���� ��
���������������1������֪�������Ȼ�ѧ����ʽ��ӳ���2���ã���ΪCH4 (g)��2NO2(g)��N2(g)��CO2(g)��2H2O(g) ��H ����867 kJ/mol
��2����ߵ��������ת���ʼ�ʹ��Ӧ������У�������������ԭ�����ɲ�ȡ����NH3��Ũ�Ȼ��С��Ӧ��ϵ��ѹǿ�ͷ�Ӧ��ϵ���¶ȵȴ�ʩ��
��3����Ӧ���з�Ӧ��ΪNO2��Na2SO3������֮һ��N2������������ԭ��Ӧ���ۣ����ƶ���һ����ΪNa2SO4��ѧ����ʽ��2NO2 + 4 Na2SO3 = N2 + 4 Na2SO4��������11.2 L N2����״�����������ʵ���Ϊ0.5mol������NO2�����ʵ���Ϊ1mol,�Ӷ��ɼ��������ClO2������Ϊ67.5g;
��4�����ݻ�ѧƽ���еġ��ȹ���ƽ�����ɣ��¶ȸߵ��ȴ�ƽ�⣬�����������ʿ죬�ȴ�ƽ�⣬����T2>T1,S1>S2,���¶����ߣ�CO��Ũ������˵������ƽ�������ƶ��������Ƿ��ȷ�Ӧ��H<0��
���㣺�����˹���ɵ�Ӧ�á�ƽ���ƶ�����������Ĺ�ϵ����ѧ����ʽ���ƶϼ����㡢��ѧƽ��ͼ��ķ�������

 �ݾ�ѵ������ϵ�д�
�ݾ�ѵ������ϵ�д� С����ȫ�ܼ��ϵ�д�
С����ȫ�ܼ��ϵ�д�I. ����H2��Cl2�����100 mL�������������һ����������Ϊ45mL������ʹ�������巢����Ӧ��ָ���������������Ϊ mL��Ϊ��˵����Ӧ��������H2��Cl2��ʣ�࣬ʹ����ͨ��10 mLˮ����ʹʣ����������ָ��������
��1������ʣ��___mL��֤����___ʣ�࣬������_____��
��2������Һ��___���ʣ�֤����___ʣ�࣬������________________________��
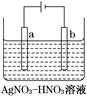
II.һλͬѧ�����һ����Ũ�����KMnO4������ȡ�����������Ƚ�������ⵥ�ʵ�������ǿ������װ�ã���ͼ��ʾ����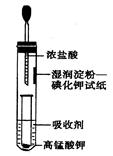
��1��������Һ������Cl2����________��
| A������ʳ��ˮ | B��Na2SO4��Һ |
| C��NaOH��Һ | D��Ũ���� |
��3��ʵ������ȡ���������ӷ���ʽ__________________________��
��ҵ����̼���̿�Ϊ��Ҫԭ������MnO2�Ĺ����������£�
�й��������↑ʼ�����ͳ�����ȫ��pH���±���
| �������� | Al��OH��3 | Fe��OH��3 | Fe��OH��2 | Cu��OH��2 | Pb��OH��2 | Mn��OH��2 |
| ��ʼ������pH | 3.3 | 1.5 | 6.5 | 4.2 | 8.0 | 8.3 |
| ������ȫ��pH | 5.2 | 3.7 | 9.7 | 6.7 | 8.8 | 9.8 |
��ش��������⣺
��1�����ǰ��̼���̿�����������_________________________________��
��2����������Һ�к���Mn2����SO42-������������Fe2����Fe3����Al3����Cu2����Pb2���ȣ�����ӹ������£�
�ټ���MnO2��Fe2���������䷴Ӧ�����ӷ���ʽΪ___________________________________��
�ڼ���CaO����Һ��pH����5.2��6.0������ҪĿ����_____________________________��
�ۼ���BaS����ȥCu2����Pb2�����ټ���NaF��Һ����ȥ________��
��3������ҺA�л��յ���Ҫ������________�������ʳ��������ʡ�
��4��MnO2��Ʒ�к�������Mn3O4��������ϡ���ᴦ��������ת��ΪMnSO4��MnO2��Ȼ��������������Mn2��ת��ΪMnO2���Ƶ�����MnO2��д��Mn3O4��ϡ���ᷴӦ�Ļ�ѧ����ʽ��______________________________________��