��Ŀ����
����Ŀ�����ʵ����Ǹ��л�ѧ���õ�������������������йؼ��㣺
��1��3.4gNH3���________molH��
��2����״���£��������CO��CO2��������Ϊ________��
��3��100mLAl2(SO4)3��Һ��c(Al3+)=0.20molL��1����c(SO42-)=_______��
��4�����ʵ���Ũ����ͬ��NaCl��MgCl2��AlCl3��Һ�зֱ����������AgNO3��Һ�У����ɳ�����������ȣ�������Һ�������Ϊ____________��
��5����״����VL�����ܽ���1Lˮ�У�ˮ���ܶȽ���Ϊ1g/mL����������Һ���ܶ�Ϊ��g/mL�������Һ�����ʵ����ʵ���Ũ��Ϊ__________��
���𰸡�0.6 7:11 0.3 mol/L 6:3:2 ![]() mol/L
mol/L
��������
(1)����n=![]() ���㰱�������ʵ�������ԭ�����ʵ���Ϊ�������ӵ�3����
���㰱�������ʵ�������ԭ�����ʵ���Ϊ�������ӵ�3����
(2)��״���£��������CO��CO2�����ʵ�����ȣ����m=nM�����������֮�ȣ�
(3)��Һ��c(SO42-)=![]() c(Al3+)���㣻
c(Al3+)���㣻
(4)���ɳ���AgCl��������ȣ�˵����������Һ�������������ʵ�����ȣ�����������Ϊ3mol������������ʵ������ٸ���V=![]() ���㣻
���㣻
(5)���ݰ���������������ʰ��������ʵ�����������Һ���������ܶȼ�����Һ������������������Һ�����ʵ���Ũ�ȡ�
(1)n(H)=3n(NH3)=3��![]() =0.6mol���ʴ�Ϊ��0.6��
=0.6mol���ʴ�Ϊ��0.6��
(2)��״���£��������CO��CO2�����ʵ�����ȣ�����m=nM��֪����������֮�ȵ���Ħ������֮��=28g/mol��44g/mol=7��11���ʴ�Ϊ��7��11��
(3)Al2(SO4)3��Һ��c(SO42-)=![]() c(Al3+)=
c(Al3+)=![]() ��0.2mol/L=0.3mol/L���ʴ�Ϊ��0.3mol/L��
��0.2mol/L=0.3mol/L���ʴ�Ϊ��0.3mol/L��
(4)���ɳ���AgCl��������ȣ�˵����������Һ���������ӵ����ʵ�����ȣ�����������Ϊ3mol����n(NaCl)=3mol��n(MgCl2)=![]() =1.5mol��n(AlCl3)=
=1.5mol��n(AlCl3)=![]() =1mol����Һ��Ũ����ȣ�����V=
=1mol����Һ��Ũ����ȣ�����V=![]() ��֪��NaCl��MgCl2��AlCl3��Һ�����֮��Ϊ3mol��1.5mol��1mol=6��3��2���ʴ�Ϊ��6��3��2��
��֪��NaCl��MgCl2��AlCl3��Һ�����֮��Ϊ3mol��1.5mol��1mol=6��3��2���ʴ�Ϊ��6��3��2��
(5)��״����VL���������ʵ���Ϊ![]() =
=![]() mol������Ϊ
mol������Ϊ![]() mol��17g/mol=
mol��17g/mol=![]() g���ܽ���1Lˮ�У�������Һ������Ϊ
g���ܽ���1Lˮ�У�������Һ������Ϊ![]() g+1000g����Һ�����Ϊ��V=
g+1000g����Һ�����Ϊ��V=![]() =
=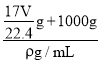 =
=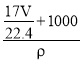 mL=
mL=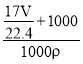 L������Һ�����ʵ����ʵ���Ũ��Ϊ��c=
L������Һ�����ʵ����ʵ���Ũ��Ϊ��c=![]() =
= =
=![]() mol/L���ʴ�Ϊ��
mol/L���ʴ�Ϊ��![]() mol/L��
mol/L��