��Ŀ����
����Ŀ��ij�����A����KAl��SO4��2��Al2O3��Fe2O3����һ�������¿�ʵ����ͼ��ʾ������֮���ת����
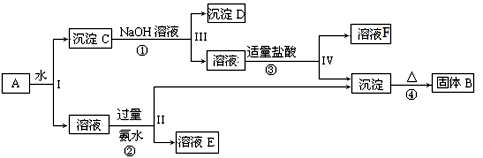
�ݴ˻ش��������⣺
(1)I��II��III��IV�IJ��ж�����Һ�ͳ����ķ����ȡ�ķ�����______��
(2)������ͼ��Ӧ��ϵ��д������B��F�������ʳɷֵĻ�ѧʽ��B______��F_____��
(3)д�����̷�Ӧ�ٵ����ӷ���ʽ_________________������B������Ӧ�Ľ����䵥��������������ڸ����·�Ӧ�Ļ�ѧ����ʽ__________________________��
(4)д�������������ʱ�����ӷ�Ӧ����ʽ_____________��
���𰸡���10�֣� ��1�����ˣ�2�֣���2��Al2O3��AlCl3����1�֣���3��Al2O3+2OH��=2AlO2��+H2O��
3Fe3O4��8Al![]() 4Al2O3+ 9Fe ����2�֣���4��AlO2��+ 4H+ = Al3+ + 2H2O��2�֣�
4Al2O3+ 9Fe ����2�֣���4��AlO2��+ 4H+ = Al3+ + 2H2O��2�֣�
��������
�����̿�֪��Al2O3��Fe2O3������ˮ�������CΪAl2O3��Fe2O3������������Ӧ�������DΪFe2O3����Ӧ�ڢ������ɵij���ΪAl��OH��3����ҺFΪ�Ȼ��ƣ������������ȷֽ���������������BΪAl2O3����Ӧ��ΪKAl��SO4��2�백ˮ�ķ�Ӧ������ҺEΪK2SO4����NH4��2SO4��NH3��H2O��Ȼ�������ʵ����ʷ������
�������Ϸ�����֪BΪAl2O3��CΪAl2O3��Fe2O3��DΪFe2O3����ҺEΪK2SO4����NH4��2SO4��NH3��H2O����ҺFΪ�Ȼ��ƣ���
��1�����벻���Թ������Һ�ķ���Ϊ���ˣ����Ԣ��IJ��ж�����Һ�ͳ����ķ��뷽��Ϊ���ˣ�
��2��������������֪��BΪAl2O3��FΪNaCl��
��3����Ӧ�������������������Ʒ�Ӧ�������ӷ���ʽΪAl2O3+2OH����2AlO2��+H2O������B������Ӧ�Ľ���������������������������ڸ����·������ȷ�Ӧ�����ɵ�����������������Ӧ����ʽΪ3Fe3O4��8Al![]() 4Al2O3+9Fe��
4Al2O3+9Fe��
��4��ƫ�������������ᷴӦ���������Ӻ�ˮ�����ӷ�Ӧ����ʽΪAlO2��+4H+��Al3++2H2O��

 ��ĩ1�����ʽ���������ϵ�д�
��ĩ1�����ʽ���������ϵ�д�