��Ŀ����
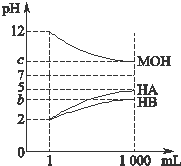 Ϊ�о�HA��HB��Һ��MOH������Ե����ǿ����ij��ѧѧϰС�����������ʵ�飺�����£���pH=2����������ҺHA��HB��pH=12�ļ���ҺMOH��1mL���ֱ��ˮϡ�͵�1000mL����pH�ı仯����Һ����Ĺ�ϵ��ͼ��ʾ�������������ݣ���ش��������⣺
Ϊ�о�HA��HB��Һ��MOH������Ե����ǿ����ij��ѧѧϰС�����������ʵ�飺�����£���pH=2����������ҺHA��HB��pH=12�ļ���ҺMOH��1mL���ֱ��ˮϡ�͵�1000mL����pH�ı仯����Һ����Ĺ�ϵ��ͼ��ʾ�������������ݣ���ش��������⣺��1��HAΪ
��2����c=9����
��ϡ��ǰHB��Һ��MOH��Һ�������Ϻ���Һ��c��B-��
��ϡ�ͺ��������Һ�У���ˮ����������ӵ�Ũ�ȵĴ�С˳��Ϊ
�۽�ϡ�ͺ��HA��Һ��MOH��Һȡ�������ϣ���������Һ��c��A-����c��M+���Ĵ�С��ϵΪ
��3����b+c=14����MOHΪ
���㣺�Ƚ�ǿ������ʵ�ʵ��
ר�⣺����ƽ������Һ��pHר��
��������1��pH=a��ǿ�ᣬϡ��10n������Һ��pH=a+n��pH=a�����ᣬϡ��10n������Һ��pH����a��a+n֮�䣮�ݴ˿�ȷ����
��2�����ȸ���c=9�ж�MOHΪǿ�����Һ��Ϻ�����HB��������Һ��ʾ���ԣ����ݵ���غ��ж�����Ũ�ȴ�С��
������Һ��ˮ����������Ũ�ȵ���ˮ�����������Ũ�ȣ�����Һ��������Ϊˮ���������ӣ����ݼ���Һ��pHֵ����������Ũ�ȣ�Ȼ���������
�۸���ͼ��֪HAΪǿ�ᣬMOHΪǿ��ֱ��ˮϡ�͵�1000mL����Һ����������������Ũ����ȣ�
��3������b+c=14��b��5�ж�c����ֵ��9�Ĵ�С���Ӷ��ж�MOH��ǿ����������ϡ�ͺ����Һ��MOH��Һ�е�������������HB�е�������Ũ����ȿ�֪�����ߵ���̶���ͬ�����Ե������Ϻ���Һ��ʾ���ԣ�
��2�����ȸ���c=9�ж�MOHΪǿ�����Һ��Ϻ�����HB��������Һ��ʾ���ԣ����ݵ���غ��ж�����Ũ�ȴ�С��
������Һ��ˮ����������Ũ�ȵ���ˮ�����������Ũ�ȣ�����Һ��������Ϊˮ���������ӣ����ݼ���Һ��pHֵ����������Ũ�ȣ�Ȼ���������
�۸���ͼ��֪HAΪǿ�ᣬMOHΪǿ��ֱ��ˮϡ�͵�1000mL����Һ����������������Ũ����ȣ�
��3������b+c=14��b��5�ж�c����ֵ��9�Ĵ�С���Ӷ��ж�MOH��ǿ����������ϡ�ͺ����Һ��MOH��Һ�е�������������HB�е�������Ũ����ȿ�֪�����ߵ���̶���ͬ�����Ե������Ϻ���Һ��ʾ���ԣ�
���
�⣺��1����ͼ��֪��pH=2����������ҺHA��HB��1mL���ֱ��ˮϡ�͵�1000mL����ҺHA��pH=5������3����λ������HAΪǿ�
��ҺHB��pHֵ�仯С��3����λ������HBΪ���ᣬ
�ʴ�Ϊ��ǿ������
��2������c=9��pH=12�ļ���ҺMOH1mLϡ��1000������Һ��pH�仯��3����λ��˵��MOHΪǿ���pH=2������HB��pH=12��ǿ����ҺMOH�������ϣ�HB��������Һ��ʾ���ԣ�c��H+����c��OH-�������ݵ���غ��֪��c��B-����c��M+����
�ʴ�Ϊ������
��pH=5��HA��Һ��c��H+��ˮ=c��OH-��ˮ=
=10-9mol/L������ˮ�����������Ũ��c��H+��ˮ=10-9mol/L��
pH=b��HB��Һ��c��H+��ˮ=c��OH-��ˮ=
=10-��14-b��mol/L��b��5������10-��14-b��mol/L��10-9mol/L��
pH=9��MOH��Һ�У���Һ�е�������Ϊˮ����ģ���c��H+��ˮ=1��10-9 mol/L��
����ˮ�����������Ũ��˳��Ϊ��MOH�THA��HB��
�ʴ𰸰���HA=MOH��HB��
pH=12��MOH����Һ1mL����ˮϡ�͵�1000mL��pH=9��pH����3����λ��MOHΪǿ���ϡ�ͺ��HA��Һ��MOH��Һȡ�������ϣ�����ǡ����ȫ��Ӧ����ǿ��ǿ���Σ���Һ�����ԣ����ݵ���غ��֪��c��A-��=c��M+����
�ʴ�Ϊ�����ڣ�
��3����b+c=14������b��5����c=14-b��15-5=9��pH=12������������Һ��c��OH-��=0.01mol/L��0.01mol/L������������Һϡ��1000������Һ��pH��9��c��OH-����10-5 mol/L��˵��MOH�����
��pH=c��MOH��Һ��c��OH-��=10c-14 mol/L=10-b mol/L��˵��ͬ�¶��£�HB��MOH�ĵ���������ͬ�����Խ�ϡ�ͺ��HB��Һ��MOH��Һȡ�������ϣ���Ӧ����Һ�����ԣ�
�ʴ�Ϊ���������ڣ�
��ҺHB��pHֵ�仯С��3����λ������HBΪ���ᣬ
�ʴ�Ϊ��ǿ������
��2������c=9��pH=12�ļ���ҺMOH1mLϡ��1000������Һ��pH�仯��3����λ��˵��MOHΪǿ���pH=2������HB��pH=12��ǿ����ҺMOH�������ϣ�HB��������Һ��ʾ���ԣ�c��H+����c��OH-�������ݵ���غ��֪��c��B-����c��M+����
�ʴ�Ϊ������
��pH=5��HA��Һ��c��H+��ˮ=c��OH-��ˮ=
| 10-14 |
| 10-5 |
pH=b��HB��Һ��c��H+��ˮ=c��OH-��ˮ=
| 10-14 |
| 10-b |
pH=9��MOH��Һ�У���Һ�е�������Ϊˮ����ģ���c��H+��ˮ=1��10-9 mol/L��
����ˮ�����������Ũ��˳��Ϊ��MOH�THA��HB��
�ʴ𰸰���HA=MOH��HB��
pH=12��MOH����Һ1mL����ˮϡ�͵�1000mL��pH=9��pH����3����λ��MOHΪǿ���ϡ�ͺ��HA��Һ��MOH��Һȡ�������ϣ�����ǡ����ȫ��Ӧ����ǿ��ǿ���Σ���Һ�����ԣ����ݵ���غ��֪��c��A-��=c��M+����
�ʴ�Ϊ�����ڣ�
��3����b+c=14������b��5����c=14-b��15-5=9��pH=12������������Һ��c��OH-��=0.01mol/L��0.01mol/L������������Һϡ��1000������Һ��pH��9��c��OH-����10-5 mol/L��˵��MOH�����
��pH=c��MOH��Һ��c��OH-��=10c-14 mol/L=10-b mol/L��˵��ͬ�¶��£�HB��MOH�ĵ���������ͬ�����Խ�ϡ�ͺ��HB��Һ��MOH��Һȡ�������ϣ���Ӧ����Һ�����ԣ�
�ʴ�Ϊ���������ڣ�
���������⿼�鶨������ǿ������ʣ��ѶȽϴؼ����pH=a��ǿ�ᣬϡ��10n������Һ��pH=a+n��pH=a�����ᣬϡ��10n������Һ��pH����a��a+n֮�䣨��Ϊ���ͣ�����3��Ϊ�ѵ���״��㣬ע�����պ����ķ���������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
����������������ϡHNO3�пɷ������·�Ӧ��3Fe3O4+28HNO3=9Fe��NO3��x+NO��+14H2O�������жϺ������ǣ�������
| A��Fe��NO3��x�е�xΪ2 |
| B��ϡHNO3�ڷ�Ӧ��ֻ���������� |
| C�������������е�������Ԫ��ȫ�������� |
| D����Ӧ��ÿ��ԭ0.3mol������������0.9mol����ת�� |
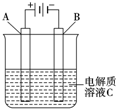
 �ҹ�Ԥ����2020��ǰ���Լ������˿ռ�վ��Ϊ��ʵ�ֿռ�վ�����ŷţ�ѭ���������������CO2���ṩ�������ҹ���ѧ�������һ��װ�ã���ͼ��ʾ����ʵ�֡�̫����һ����һ��ѧ�ܡ�ת�����ܷ�ӦΪ2CO2=2CO+O2�������й�˵����ȷ���ǣ�������
�ҹ�Ԥ����2020��ǰ���Լ������˿ռ�վ��Ϊ��ʵ�ֿռ�վ�����ŷţ�ѭ���������������CO2���ṩ�������ҹ���ѧ�������һ��װ�ã���ͼ��ʾ����ʵ�֡�̫����һ����һ��ѧ�ܡ�ת�����ܷ�ӦΪ2CO2=2CO+O2�������й�˵����ȷ���ǣ�������| A����װ������ԭ��� |
| B��X����Ӧʽ��O2+2H2O+4e-=4OH- |
| C����Ӧ��ϣ���̫����װ���еĵ������Һ������ǿ |
| D�����������ˮ��������Y����Ӧ��CO2+H2O+2e-=CO+2OH- |

 ��Ԫ���ж��ֻ��ϼۣ����γɶ��ֻ����
��Ԫ���ж��ֻ��ϼۣ����γɶ��ֻ����
 �£�N2H4���ֳ���������һ�ֿ�ȼ��Һ�壬�������������ﷴӦ�����ɵ�����ˮ���������ȼ�ϣ�
�£�N2H4���ֳ���������һ�ֿ�ȼ��Һ�壬�������������ﷴӦ�����ɵ�����ˮ���������ȼ�ϣ�