��Ŀ����
����Ŀ����ͨ�������£�����ͼ��ʾװ�����Ҷ�ȩ��OHC-CHO���Ʊ��Ҷ��ᣨH00C-COOH�������Ʊ���ӦΪ��OHC-CHO+2Cl2+2H2O��HOOC-COOH+4HCl������˵����ȷ����
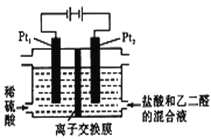
A. ÿ����0.1mol�Ҷ�ȩ��Pt1���ų�2.24L���壨��״����
B. Pt1�ĵ缫��ӦΪ��4OH--4e-=2H2O+O2��
C. ���������ṩCl-����ǿ�����Ե�����
D. ÿ�õ�lmol�Ҷ��Ὣ��2molH+������Ǩ�Ƶ�����
���𰸡�C
��������A��Pt1Ϊ�����õ��ӷ�����ԭ��Ӧ��
B��Pt1Ϊ�����õ��ӷ�����ԭ��Ӧ��
C��HCl�ǵ���ʣ�
D��Pt2Ϊ�������ݴ˽��з�����
A��Pt1Ϊ�����õ��ӷ�����ԭ��Ӧ�������ĵ缫��Ӧ����ʽΪCl2+2e-��2Cl-����Ϊ����Cl2�������Ƿų�Cl2��A����
B��Pt1Ϊ�����õ��ӷ�����ԭ��Ӧ�������ĵ缫��Ӧ����ʽΪCl2+2e-��2Cl-��B����
C��HCl�ǵ���ʣ�����ˮ��Һ�����ṩCl-����ǿ�����Ե����ã�C��ȷ��
D��Pt2Ϊ�����������ĵ缫��Ӧ����ʽΪOHC-CHO-4e-+2H2O=HOOC-COOH+4H+����ÿ�õ�1mol�Ҷ��Ὣ��4molH+������Ǩ�Ƶ����ң�D����ѡC��

����Ŀ��700��ʱ�����ݻ�Ϊ2 L�ĺ����ܱ������г���һ������CO��H2���������·�Ӧ��
CO(g)��2H2(g)![]() CH3OH(g)����Ӧ�����вⶨ�IJ������ݼ��±���
CH3OH(g)����Ӧ�����вⶨ�IJ������ݼ��±���
��Ӧʱ��/min | n(CO)/mol | n(H2)/ mol |
0 | 0.60 | 1.20 |
20 | 0.20 | |
30 | 0.40 |
����˵����ȷ����
A. ��Ӧ��20 min�ڵ�ƽ������Ϊv(H2)��0.04 mol��L��1��min��1
B. ���������������䣬�����¶ȣ�ƽ��ʱc(CH3OH)= 0.15 mol��L-1����Ӧ����H<0
C. ���������������䣬����ƽ����ϵ��ͬʱͨ��0.20 mol CO��0.20 mol H2��0.20 mol CH3OH���ﵽ��ƽ��ǰv(��)> v(��)
D. ��ͬ�¶��£�����ʼʱ�������г���1.0 mol CH3OH���ﵽƽ��ʱCH3OHת���ʴ���1/3




