��Ŀ����
13����1����ϵͳ��������������
 3��3��5��5-�ļ����飻
3��3��5��5-�ļ����飻�ڣ�CH3��2CHCH=CHCH34-��-2-��ϩ��
��2��д�����и��л���Ľṹ��ʽ��
��2��3-����-4-�һ����飺CH3CH��CH3��CH��CH3��CH��C2H5��CH2CH3��
��֧��ֻ��һ���һ�����Է���������С��������CH3CH2CH��C2H5��CH2CH3��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��
��ʵ��������ϩCH3-CH2-OH $��_{170��}^{Ũ����}$CH2-CH2��+H2O��
���Ҵ�������2CH3CH2OH+O2$��_{��}^{Cu��Ag}$2CH3CHO+2H2O��
��2-�ȱ�����ȥ��ӦCH3-CHCl-CH3+NaOH$��_{��}^{�Ҵ�}$CH3-CH=CH2��+NaCl+H2O��
����ȩ������������ͭCH3CHO+2Cu��OH��2$\stackrel{��}{��}$CH3COOH+Cu2O+2H2O��
���� ��1���ٸ�������������ԭ�����������Ҫ���ϡ�һ����һ����һ�ࡢһС����Ҳ�������������������֧�������֧����ĿҪ�֧࣬��λ�ú���֮����С������ǰͬ�ಢ�����Ӧ��һ���ߣ�
�ں��й����ŵ��л�������ʱ��Ҫѡ�������ŵ��̼����Ϊ�����������ŵ�λ����С��
��2���ٸ��л���Ϊ��������������������ԭ��Ը��л�����нṹ��ʽ����д��
�������г����һ������������ٺ���5��C���ݴ�д�����л���Ľṹ��ʽ��
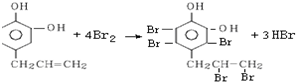
��3�����Ҵ���Ũ������ȷ�����ȥ��Ӧ��ȡ��ϩ��
���Ҵ���������������ȩ��ˮ��
��±�����ڼ�Ĵ���Һ�м��ȷ�����ȥ��Ӧ����ϩ����
����ȩ������������ͭ����������ԭ��Ӧ�������ᡢ������ͭ��ˮ��
��� �⣺��1�����������̼������7��C��Ϊ���飬��3��5��C�ϸ���2��������������Ϊ��3��3��5��5-�ļ����飬
�ʴ�Ϊ��3��3��5��5-�ļ����飻
���̼������5��C������Ϊ��ϩ����4��C����1��������2��3��C����˫������������Ϊ��4-��-2-��ϩ��
�ʴ�Ϊ��4-��-2-��ϩ��
��2����2��3-����-4-�һ����飬�����������6��Cԭ�ӣ�2��3��̼ԭ���ϸ�����1��������4��C����1���һ�����ṹ��ʽΪ��CH3CH��CH3��CH��CH3��CH��C2H5��CH2CH3��
�ʴ�Ϊ��CH3CH��CH3��CH��CH3��CH��C2H5��CH2CH3��
�������к����һ������һ�������3��λ������ֻ��һ���һ���ʽ����С�������Ľṹ��ʽΪCH3CH2CH��C2H5��CH2CH3��
�ʴ�Ϊ��CH3CH2CH��C2H5��CH2CH3��
��3����ʵ���������Ҵ��ܷ�����ȥ��Ӧ����ϩ��CH3-CH2-OH $��_{170��}^{Ũ����}$CH2-CH2��+H2O��
�ʴ�Ϊ��CH3-CH2-OH $��_{170��}^{Ũ����}$CH2-CH2��+H2O��
���Ҵ��к�-OH���ܷ���������������ȩ���䷴Ӧ����ʽΪ��2CH3CH2OH+O2$��_{��}^{Cu��Ag}$2CH3CHO+2H2O��
�ʴ�Ϊ��2CH3CH2OH+O2$��_{��}^{Cu��Ag}$2CH3CHO+2H2O��
��2-�ȱ�����NaOH���Ҵ���Һ���ȷ�����ȥ��Ӧ������ʽΪ��CH3-CHCl-CH3+NaOH$��_{��}^{�Ҵ�}$CH3-CH=CH2��+NaCl+H2O��
�ʴ�Ϊ��CH3-CHCl-CH3+NaOH$��_{��}^{�Ҵ�}$CH3-CH=CH2��+NaCl+H2O��
�ܣ��ڼ��������£���ȩ������������ͭ����������ԭ��Ӧ�������ᡢ������ͭ��ˮ����Ӧ����ʽΪ��CH3CHO+2Cu��OH��2$\stackrel{��}{��}$CH3COOH+Cu2O+2H2O��
�ʴ�Ϊ��CH3CHO+2Cu��OH��2$\stackrel{��}{��}$CH3COOH+Cu2O+2H2O��
���� ���⿼�����л����������ṹ��ʽ����д����ѧ��Ӧ����ʽ����д������ע���˻���������Ŀ��飬���ض�ѧ������֪ʶ�ļ����ѵ��������Ĺؼ�����ȷ�л��������ԭ��Ȼ�����л���Ľṹ��ʽ������ã�����������ѧ���Ĺ淶����������ע���л��ﷴӦ���ͼ���Ӧ��������Ŀ�ѶȲ���

 �����Ļ�������ҵϵ�д�
�����Ļ�������ҵϵ�д� ����������������ϵ�д�
����������������ϵ�д���
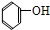 ��
��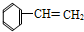 ��
��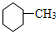 ��
��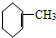 ��
��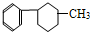 ��
��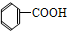 ��
��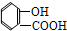 ��
��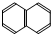 ��
��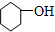 ��
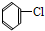
��
| A�� | �ڢۢܢݢ�� | B�� | �ڢݢ� | C�� | �ۢܢ�� | D�� | �ڢ�� |
 ��Fˮ��Һ���Լ��ԣ�����ԡ����Ի���ԣ���
��Fˮ��Һ���Լ��ԣ�����ԡ����Ի���ԣ���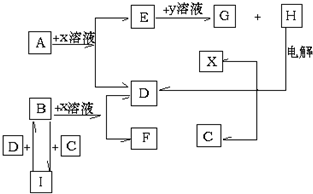
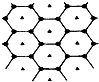 ̼��һ�ֵ���ʯī�ʲ�״�ṹ����һ̼þ���Ͳ��Ͼ�����ʯī̼ԭ�Ӳ�����þԭ�Ӳ㣬���㸩��ͼ���ò��ϵĻ�ѧʽΪMgC2��
̼��һ�ֵ���ʯī�ʲ�״�ṹ����һ̼þ���Ͳ��Ͼ�����ʯī̼ԭ�Ӳ�����þԭ�Ӳ㣬���㸩��ͼ���ò��ϵĻ�ѧʽΪMgC2�� ����Ⱥ����ܱ�������ͨ��SO2��NO2����һ��������ʹ��ӦSO2��g��+NO2��g��?SO3��g��+NO��g���ﵽƽ�⣬����Ӧ������ʱ��仯��ʾ��ͼ��ͼ��ʾ��
����Ⱥ����ܱ�������ͨ��SO2��NO2����һ��������ʹ��ӦSO2��g��+NO2��g��?SO3��g��+NO��g���ﵽƽ�⣬����Ӧ������ʱ��仯��ʾ��ͼ��ͼ��ʾ��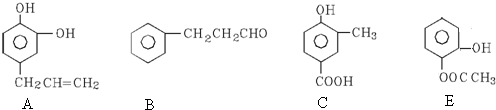
 ��
��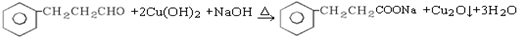 ��
��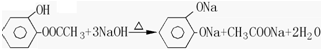 ��
��