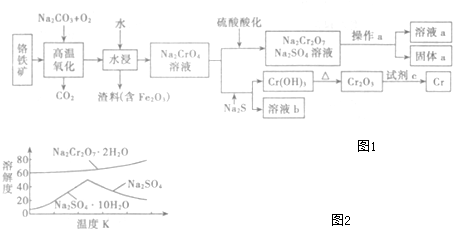
��Ŀ����
9��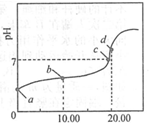 �����£���0.01mol•L-1NaOH��Һ�ζ�20.00mL0.01mol•L-1CH3COOH��Һ�����õζ�������ͼ������˵����ȷ���ǣ�������
�����£���0.01mol•L-1NaOH��Һ�ζ�20.00mL0.01mol•L-1CH3COOH��Һ�����õζ�������ͼ������˵����ȷ���ǣ�������| A�� | a��PH=2 | |
| B�� | b���Ӧ����Һ�У�c��OH-��+c��CH3COO-��=c��Na+��+c��H+�� | |
| C�� | c���ʾNaOH��Һ��CH3COOH��Һǡ����ȫ��Ӧ | |
| D�� | d����õ���Һ�У�ˮ�ĵ���̶�С��ͬ���´�ˮ�ĵ���̶� |
���� A������Ϊ���ᣬ������ȫ���룻
B��b��ʱ��Һ�����ԣ���ϵ���غ���
C��c��ʱ��Һ�����ԣ���������������ǡ����ȫ��Ӧ������ҺӦ�ʼ��ԣ�
D��d��ʱ��Һ�ʼ��ԣ������ǡ���кͣ������ɴ����ƴٽ�ˮ�ĵ��룮
��� �⣺A������Ϊ���ᣬ������ȫ���룬��0.01mol•L-1 CH3COOH��ҺpH��2����A����
B����Һ�д��ڵ���غ㣺c��Na+��+c��H+��=c��OH-��+c��CH3COO-������B��ȷ��
C��������������Ʒ�Ӧ���ɴ����ƣ���������ǿ����������ˮ��Һ�ʼ��ԣ������ǡ�÷�Ӧʱ��ҺӦ�óʼ��ԣ���C����Һ�����ԣ�˵�����������C����
D��d��ʱ��Һ�ʼ��ԣ�����ǡ���кͣ���ȫ��Ӧ���ɴ����ƣ�ˮ��ʼ��ԣ��ٽ�ˮ�ĵ��룬��d��ˮ�ĵ���̶ȴ���ͬ���´�ˮ�ĵ���̶ȣ���D����
��ѡB��
���� ������NaOH�ζ�CH3COOH�ĵζ�����Ϊ���壬���������ˮ�⡢��Һ����Ũ�ȵĴ�С�Ƚϵȣ��Ѷ��еȣ�ע������Ũ�ȴ�С�Ƚ��е���غ㣬����Ӱ��������ʵĵ��������ˮ������أ�ע����ػ���֪ʶ�Ļ��ۣ��ѶȲ���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
12������CH3COOH��������״��ϩ���Ļ���������������������Ϊa����̼�����������ǣ�������
| A�� | $\frac{1-a}{7}$ | B�� | $\frac{3}{4}$a | C�� | $\frac{6}{7}$��1-a�� | D�� | $\frac{12}{13}$��1-a�� |
13�������£����ݻ��̶����ܱ������У���������̿��һ����������̼������������ȷ�Ӧ������CO������ѡ���У�һ�����Ϸ�Ӧ�ﵽ��ѧƽ��ʱ�����ѡ���ǣ�������
| A�� | ѹ������ﵽ��ƽ��ʱ��CO��Ũ������ı�����CO2��Ũ������ı������� | |
| B�� | ���ʹ�ϵ��2v��CO2��=v��CO�� | |
| C�� | ת�Ƶ��������¶����߶����� | |
| D�� | �����ܶ����Ž�̿�����Ӷ����� |
10�����������У������ڴ�����ǣ�������
| A�� | C4H9OH | B�� | C6H5CH2OH | C�� | C6H5OH | D�� | 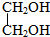 |
14��������Ԫ��R��Q��M��T��Ԫ�����ڱ��е����λ���������֪Rԭ�����������������������֮��Ϊ2��1��
��1���˵ĺ�Һ�к���T�ļ����ӣ������ӽṹʾ��ͼΪ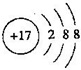 �� Ԫ��M��Ԫ�����ڱ��е�λ���ǵ������ڵ�VIA�壮
�� Ԫ��M��Ԫ�����ڱ��е�λ���ǵ������ڵ�VIA�壮
��2��R����������������Ļ�ѧ�������ǹ��ۼ���ѡ����ӡ����ۡ�����
��3������ʱ��Q������������Ӧˮ�����Ũ��Һ�뵥��R��Ӧ�Ļ�ѧ����ʽΪ4HNO3��Ũ��+C$\frac{\underline{\;\;��\;\;}}{\;}$4NO2��+CO2��+2H2O���þ���Ļ�ѧʽ��ʾ����
��4����һ�������¼ס��ҡ���������ת������$\stackrel{+X}{��}$��$��_{���Ϸ�Ӧ}^{+X}$���������м��ǵ��ʣ��ҡ���Ϊ�����x�Ǿ��������Ե���ɫ���嵥�ʣ���Ļ�ѧ��ɲ������Ǣܣ�ѡ����ţ���
��R ��Q2 ��M ��T2
��5��Ԫ��T�ĺ�����HTO����Ư����д��HTO�ĵ���ʽ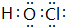 ��
��
| R | Q | ||
| M | T |
 �� Ԫ��M��Ԫ�����ڱ��е�λ���ǵ������ڵ�VIA�壮
�� Ԫ��M��Ԫ�����ڱ��е�λ���ǵ������ڵ�VIA�壮��2��R����������������Ļ�ѧ�������ǹ��ۼ���ѡ����ӡ����ۡ�����
��3������ʱ��Q������������Ӧˮ�����Ũ��Һ�뵥��R��Ӧ�Ļ�ѧ����ʽΪ4HNO3��Ũ��+C$\frac{\underline{\;\;��\;\;}}{\;}$4NO2��+CO2��+2H2O���þ���Ļ�ѧʽ��ʾ����
��4����һ�������¼ס��ҡ���������ת������$\stackrel{+X}{��}$��$��_{���Ϸ�Ӧ}^{+X}$���������м��ǵ��ʣ��ҡ���Ϊ�����x�Ǿ��������Ե���ɫ���嵥�ʣ���Ļ�ѧ��ɲ������Ǣܣ�ѡ����ţ���
��R ��Q2 ��M ��T2
��5��Ԫ��T�ĺ�����HTO����Ư����д��HTO�ĵ���ʽ
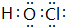 ��
��
1�����й����л�ѧ�����ƻ����ǣ�������
�ٵ����� ����������ľ̿���� �۾ƾ�����ˮ ��HCl��������ˮ��MgCl2�ܽ���ˮ ��NaCl�ۻ���
�ٵ����� ����������ľ̿���� �۾ƾ�����ˮ ��HCl��������ˮ��MgCl2�ܽ���ˮ ��NaCl�ۻ���
| A�� | ȫ�� | B�� | �ڢۢܢݢ� | C�� | �ܢݢ� | D�� | �ݢ� |
18�� ������������к��г���������������һЩ�ɷֿ���Ϊ��ͬϵ����磺�Դ�˳���Ʋ������d��e��f���ȣ��ڸ�ϵ�л������У�̼�����ٷֺ����ǣ�������
������������к��г���������������һЩ�ɷֿ���Ϊ��ͬϵ����磺�Դ�˳���Ʋ������d��e��f���ȣ��ڸ�ϵ�л������У�̼�����ٷֺ����ǣ�������
 ������������к��г���������������һЩ�ɷֿ���Ϊ��ͬϵ����磺�Դ�˳���Ʋ������d��e��f���ȣ��ڸ�ϵ�л������У�̼�����ٷֺ����ǣ�������
������������к��г���������������һЩ�ɷֿ���Ϊ��ͬϵ����磺�Դ�˳���Ʋ������d��e��f���ȣ��ڸ�ϵ�л������У�̼�����ٷֺ����ǣ�������| A�� | 100% | B�� | 56% | C�� | 97.3% | D�� | 93.8% |
19�������£���10mL0.1mol•L-1��HCl��Һ����μ���0.1mol•L-1��NH3•H2O��Һ����pH������ͼ��a��b��c�����pHΪʵ������ã������й�˵����һ��������ǣ�������
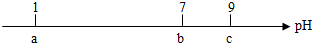
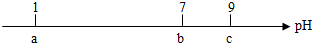
| A�� | �����£�0.1mol•L-1��HCl��Һ��pH=1 | |
| B�� | ��pH=7ʱ������NH3•H2O��Һ���������10mL | |
| C�� | ��7��pH��9ʱ����Һ��c��NH4+����c��Cl-�� | |
| D�� | �����μ�0.1mol•L-1��NH3•H2O��Һ����Һ��pH���տ��Ա仯��13 |