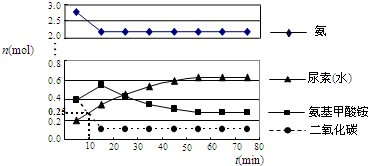
��Ŀ����
17����һ�ֹ㷺Ӧ�����������ҵ��Ʒ�ϵĸ߷���Ϳ���ǰ���������ͼ�����ģ�ͼ��M��C3H4O����A�����Է���������Ӧ��N��M�ķ�����̼ԭ������ȣ�A��������һ��ȡ��λ�������֣�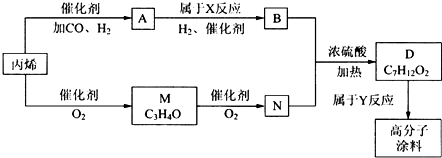
��1����д���������ʵĽṹ��ʽ��ACH3CH2CH2CHO��MCH2=CH-CHO��
��2��д����ӦN+B��D�Ļ�ѧ����ʽ��CH2=CH-COOH+CH3CH2CH2CH2OH$��_{��}^{Ũ����}$CH2=CH-COOCH2CH2CH2CH3+H2O��
��3��д����Ӧ���ͣ�X�ӳɷ�Ӧ��Y�Ӿ۷�Ӧ��
���� M����ʽ��C3H4O���ɷ���������Ӧ�������к���ȩ��-CHO���䲻���Ͷ�Ϊ$\frac{2��3+2-4}{2}$=2���ʷ����л�����C=C˫������M�Ľṹ��ʽΪCH2=CH-CHO��M��N�ķ�����̼ԭ������ͬ��M����������N����NΪCH2=CH-COOH��N��B��Ӧ��Ũ���ᡢ��������������D��C7H12O2����DΪ����BΪ������D�ķ���ʽ��֪��BΪC4H10O��A�ɷ���������Ӧ��A�к���ȩ��-CHO��A�������ϵ�һ�ȴ�����3�֣���AΪCH3CH2CH2CHO��BΪCH3CH2CH2CH2OH��DΪCH2=CH-COOCH2CH2CH2CH3���ݴ˽��
��� �⣺M����ʽ��C3H4O���ɷ���������Ӧ�������к���ȩ��-CHO���䲻���Ͷ�Ϊ$\frac{2��3+2-4}{2}$=2���ʷ����л�����C=C˫������M�Ľṹ��ʽΪCH2=CH-CHO��M��N�ķ�����̼ԭ������ͬ��M����������N����NΪCH2=CH-COOH��N��B��Ӧ��Ũ���ᡢ��������������D��C7H12O2����DΪ����BΪ������D�ķ���ʽ��֪��BΪC4H10O��A�ɷ���������Ӧ��A�к���ȩ��-CHO��A�������ϵ�һ�ȴ�����3�֣���AΪCH3CH2CH2CHO��BΪCH3CH2CH2CH2OH��DΪCH2=CH-COOCH2CH2CH2CH3��
��1��������������֪��AΪCH3CH2CH2CHO��MΪCH2=CH-CHO��
�ʴ�Ϊ��CH3CH2CH2CHO��CH2=CH-CHO��
��2��N+B��D��Ӧ��CH2=CH-COOH��CH3CH2CH2CH2OH����������Ӧ����CH2=CH-COOCH2CH2CH2CH3����Ӧ����ʽΪCH2=CH-COOH+CH3CH2CH2CH2OH$��_{��}^{Ũ����}$CH2=CH-COOCH2CH2CH2CH3+H2O��
�ʴ�Ϊ��CH2=CH-COOH+CH3CH2CH2CH2OH$��_{��}^{Ũ����}$CH2=CH-COOCH2CH2CH2CH3+H2O��
��3��Aת������B��CH3CH2CH2CHO�����������ӳɷ�Ӧ����CH3CH2CH2CH2OH��D�����Ӿ۷�Ӧ���ɸ߷���Ϳ�ϣ�
�ʴ�Ϊ���ӳɷ�Ӧ���Ӿ۷�Ӧ��
���� ���⿼���л�����ƶϣ��Ѷ��еȣ�����B�ķ���ʽ����Ӧ��Ϣ���ƶ�B�Ľṹ�ǽ���Ĺؼ�������D�ķ���ʽ��N�Ľṹ��ʽ�ж�BΪ��������ȷ��A�Ľṹ��

| ������ | ����� | ����� | �ǵ���� | |
| A | ����ˮ | ��ˮ | ������ | �������� |
| B | ���� | ���� | ���� | �ɱ� |
| C | ���� | ���� | �� | ̼��� |
| D | ��ʯ�� | ���� | �Ȼ�ͭ | ̼���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
��2��Fe��Mn��Ԫ�صIJ��ֵ��������������±���
| Ԫ �� | Mn | Fe | |
| ������ /kJ•mol-1 | I1 | 717 | 759 |
| I2 | 1509 | 1561 | |
| I3 | 3248 | 2957 | |
| A�� | ��ɫ | B�� | ״̬ | C�� | ���� | D�� | ԭ�Ӻ� |
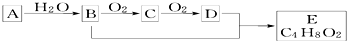
| A�� | ����A�ͼ����ѡ�����Ը��������Һ | |
| B�� | B��D����������Ʒ�Ӧ | |
| C�� | ����C�Ľṹ��ʽΪCH3CHO | |
| D�� | B+D��E�Ļ�ѧ����ʽΪ��CH3CH2OH+CH3COOH$��_{��}^{Ũ����}$CH3COOC2H5 |
| A�� | M��N��Q�������ʵ�Ũ��һ����� | |
| B�� | M��Nȫ�������Q | |
| C�� | ��Ӧ�������ɷֵİٷ���ɲ��ٱ仯 | |
| D�� | ��Ӧ�Ѿ�ֹͣ |
| A�� | ������ԭ��Ӧ�ı�����Ԫ�ػ��ϼ۷����˱仯 | |
| B�� | ������ԭ��Ӧ�ı����Ƿ�Ӧ���е��ӵ�ת�ƣ���ʧ��ƫ�ƣ� | |
| C�� | �������ڷ�Ӧ�б����� | |
| D�� | ������Ӧһ�����ڻ�ԭ��Ӧ���� |