��Ŀ����
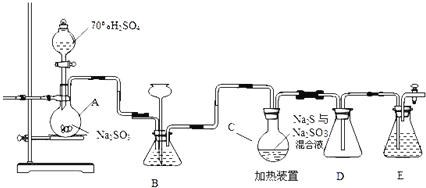
����Ŀ����ҵ�ϳ����ú����ˮ����Na2S2O35H2O��ʵ���ҿ�������װ�ã���ȥ���ּӳ�������ģ�����ɹ��̡�
��ƿC�з�����Ӧ���£�
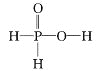
Na2S��aq��+H2O��l��+SO2��g��=Na2SO3��aq��+H2S��aq������ ����
2H2S��aq��+SO2��g��=3S��s��+2H2O��l�������������������� ����
S��s��+Na2SO3��aq��![]() Na2S2O3��aq������������������ ����
Na2S2O3��aq������������������ ����
��1��װ��A�з����Ļ�ѧ��Ӧ����ʽΪ ______
��2��Ϊ��߲�Ʒ���ȣ�Ӧʹ��ƿC��Na2S��Na2SO3ǡ����ȫ��Ӧ������ƿC��Na2S��Na2SO3���ʵ���֮��Ϊ ______ ��
��3��װ��B������֮һ�ǹ۲�SO2���������ʣ����е�Һ�����ѡ�� ______
a������ˮ����b������Na2SO3��Һ����c������NaHSO3��Һ�� d������NaHCO3��Һ
ʵ���У�ΪʹSO2����������ƿC�����õIJ����� ______ ��
��4����֪��Ӧ������Խ���������ƿC�з�Ӧ�ﵽ�յ�������� ______ ��װ��E������Ϊ ______
��5����Ӧ��ֹ����ƿC�е���Һ������Ũ����������Na2S2O35H2O�����п��ܺ���Na2SO3��Na2SO4�����ʣ����������Լ����ʵ�飬����Ʒ���Ƿ����Na2SO4����Ҫ˵��ʵ�����������ͽ��ۣ� ______ ��
��֪��Na2S2O35H2O�����ֽ⣺S2O32+2H+=S��+SO2��+H2O����ѡ����Լ���ϡ���ᡢϡ���ᡢϡ���ᡢBaCl2��Һ��AgNO3��Һ��
���𰸡�Na2SO3+H2SO4=Na2SO4+SO2��+H2O 2��1 c ���Ƶμ�������ٶ� ��Һ����壨�������ʧ�� ����SO2��ֹ��Ⱦ���� ȡ������Ʒ��������ϡ�����У����ã�ȡ�ϲ���Һ������˺�ȡ��Һ�����μ�BaCl2��Һ�������ְ�ɫ������˵������Na2SO4����
��������
Aװ����Na2SO3�����ᷴӦ�Ʊ�SO2��Bװ�ÿɹ۲�SO2�����٣�Cװ����SO2��Na2SO3��Na2S�Ļ��Һ��Ӧ�Ʊ�Na2S2O3��Dװ������ȫƿ����������Eװ������SO2������Ⱦ������
��1������װ��ͼ��֪��A�еķ�ӦΪŨ�������������Ʒ�Ӧ���ɶ�������Ӧ�Ļ�ѧ����ʽΪ��Na2SO3+H2SO4=Na2SO4+SO2��+H2O��
�ʴ�Ϊ��Na2SO3+H2SO4=Na2SO4+SO2��+H2O��
��2��C�з����ķ�ӦΪNa2S��aq��+H2O��l��+SO2��g��=Na2SO3��aq��+H2S��aq������
2H2S��aq��+SO2��g��=3S��s��+2H2O��l������
S��g��+Na2SO3��aq��![]() Na2S2O3��aq������
Na2S2O3��aq������
ΪʹNa2S��Na2SO3ǡ����ȫ��Ӧ����������2+����+������3���õ��ܷ�ӦΪ2Na2S��aq��+Na2SO3��aq��+3SO2��g��![]() 3Na2S2O3��aq������C��Na2S��Na2SO3���ʵ���֮��Ϊ2��1��
3Na2S2O3��aq������C��Na2S��Na2SO3���ʵ���֮��Ϊ2��1��
�ʴ�Ϊ��2��1��
��3��a���������������ˮ��������ˮ���ܹ۲�SO2���������ʣ�b�Na2SO3��Һ����SO2�����ܹ۲�SO2���������ʣ�c��������������ڱ���NaHSO3��Һ����ͨ���۲����ݹ۲�SO2���������ʣ�d�����NaHCO3��Һ���������Ӧ����CO2�����ܹ۲�SO2���������ʣ���ѡc��ΪʹSO2����������ƿC��ͨ�����Ƶμ�������ٶȣ����Կ��Ʋ���������������ʣ�
�ʴ�Ϊ��c�����Ƶμ�������ٶȣ�
��4����֪��Ӧ��III������������ʵ�������C�г��ֻ��ǣ���ƿC�з�Ӧ�ﵽ�յ㷢����ӦΪ�����������Ʒ�Ӧ������������ƣ���Ӧ������Ϊ��Һ����壨�������ʧ������Ӧβ������δ��Ӧ�Ķ����������壬����Ⱦ����������Ҫ��Eװ������SO2��ֹ��Ⱦ������
�ʴ�Ϊ����Һ����壨�������ʧ��������SO2��ֹ��Ⱦ������
��5������Ʒ���Ƿ����Na2SO4���������Ƿ����SO42-������Na2S2O35H2O�����ֽ���ֵ���ɫ���ǣ�����Ҫ�ȼ����������ų����ţ���ʵ�����������ͽ���Ϊ��ȡ������Ʒ��������ϡ�����У����ã�ȡ�ϲ���Һ������˺�ȡ��Һ�����μ�BaCl2��Һ�������ְ�ɫ������˵������Na2SO4���ʣ�
�ʴ�Ϊ��ȡ������Ʒ��������ϡ�����У����ã�ȡ�ϲ���Һ������˺�ȡ��Һ�����μ�BaCl2��Һ�������ְ�ɫ������˵������Na2SO4���ʡ�