��Ŀ����
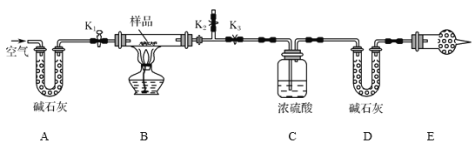
����Ŀ����һ�� NaCl��Na2CO3��10H2O��NaHCO3�Ļ���ijͬѧ�����ͼ��ʾ��ʵ��װ�ã�ͨ��������Ӧ������CO2��H2O����������ȷ���û�����и���ֵ�����������
��1��ʵ�鲽�裺
����ͼ(�г�����δ����)��װ��ʵ��װ�ú����Ƚ��еIJ�����_________________��
����ȡ��Ʒ�����������Ӳ�ʲ������У�����װŨ�����ϴ��ƿC��������װ��ʯ�ҵ�U�ι�D��������
������K1��K2���ر�K3������������������ӣ���Ŀ����______________��
���رջ���K1��K2����K3����ȼ�ƾ��Ƽ��������ٲ������塣װ��B�з�����Ӧ�Ļ�ѧ����ʽΪ______________________��_______________��
������K1������������������ӣ�Ȼ�����װ�ã��ٴγ���ϴ��ƿC��������U�ι�D��������
��2�����ڸ�ʵ�鷽������ش��������⡣
�����ʵ����û��Eװ�ã���ᵼ�²������NaHCO3������________(����ƫ��������ƫС��������Ӱ����)��
������Ʒ����Ϊw g����Ӧ��C��Dװ�����ӵ������ֱ�Ϊm1 g��m2 g����������Na2CO3��10H2O����������Ϊ________(�ú�w��m1��m2�Ĵ���ʽ��ʾ)��
���𰸡����װ�������� ��ȥװ���е�ˮ�����Ͷ�����̼ 2NaHCO3![]() Na2CO3��H2O����CO2�� Na2CO3��10H2O
Na2CO3��H2O����CO2�� Na2CO3��10H2O![]() Na2CO3��10H2O�� ƫ��
Na2CO3��10H2O�� ƫ�� ![]() ��100%
��100%
��������
���������Ȼ����H2O��g����CO2�����壬Ӧ��C��D�зֱ����գ��ɸ���������ʿ�֪��Ӧ������ˮ�������ն�����̼����C�еĸ������ˮ��������CO2����D�����أ�NaHCO3�ֽ������CO2�������������NaHCO3��������C�����أ�Na2CO310H2O�ֽ������H2O���Ѿ�֪����NaHCO3�ֽ������H2O�������������Na2CO310H2O���������Ӷ����NaCl����������Ӧ��ʵ��ǰ�뷨�ų�װ���еĿ������ؼ�����Ӧ���ų�B�еĿ��������Դ���K1��K2���ر�K3���ͳ�Ϊ�����Ĺؼ�������ͨ������Ϊ�˸ϳ�Ч�����ã�E�м�ʯ�ҿɷ�ֹ�������е�H2O��g����CO2����װ��DӰ��ʵ�飬�ݴ˽��
��1������ʵ��ԭ����֪��ʵ����Ҫͨ������Dװ���ڼ�ʯ�ҵ����أ��������ɵĶ�����̼��������ͨ������Cװ��װ�ã��������ɵ�ˮ���������ʰ�ͼ(�г�����δ����)��װ��ʵ��װ�ú����Ƚ��еIJ����Ǽ��װ�õ������ԣ�
��װ�����п���������ˮ�����Ͷ�����̼��Ӱ��ˮ�����Ͷ�����̼�����IJⶨ������K1��K2���ر�K3������������������ӣ���Ŀ���dz�ȥװ���е�ˮ�����Ͷ�����̼��
�ܺ�NaCl��Na2CO3��10H2O��NaHCO3�Ļ�������ʱ��̼�����Ʒֽ�����̼���ơ�������̼��ˮ��̼���ƾ���ʧȥ�ᾧˮ����̼���ƣ���Ӧ�Ļ�ѧ����ʽΪ2NaHCO3![]() Na2CO3��H2O����CO2����Na2CO3��10H2O
Na2CO3��H2O����CO2����Na2CO3��10H2O![]() Na2CO3��10H2O����
Na2CO3��10H2O����
��2���ٸ������ʢ�ŵ��Ǽ�ʯ�ң���ʯ�������տ����е�ˮ�����Ͷ�����̼�����Ը���ܵ������Ƿ�ֹ�����е�CO2��ˮ��������Ӱ��ⶨ���������ȥEװ�ã���ⶨ��̼�����Ƶ�����ƫ��
������Ʒ����Ϊw g����Ӧ��C��Dװ�����ӵ������ֱ�Ϊm1 g��m2 g��Dװ�������ӵ�����Ϊ������̼��������̼�����Ʒֽ����ɵ�ˮ����������Ϊx g����
2NaHCO3![]() Na2CO3��H2O����CO2��
Na2CO3��H2O����CO2��
18g 44g
x m2 g
���x��18m2/44
װ��C���յ���ˮ����������̼�����Ʒֽ����ɵĺ�ʮˮ̼���Ʒֽ����ɵģ�ʮˮ̼���Ʒֽ����ɵ�ˮ����������Ϊ(m1g��18m2/44)g�������Na2CO3��10H2O![]() Na2CO3��10H2O����֪ʮˮ̼���Ƶ�����Ϊ
Na2CO3��10H2O����֪ʮˮ̼���Ƶ�����Ϊ![]() ������ʮˮ̼���Ƶ���������Ϊ
������ʮˮ̼���Ƶ���������Ϊ![]() ��
��