��Ŀ����
6��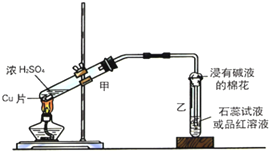 ʵ���ҳ�������װ��������ͭ��Ũ���ᷴӦ��һϵ��ʵ�飮
ʵ���ҳ�������װ��������ͭ��Ũ���ᷴӦ��һϵ��ʵ�飮��1������ʲô������ж�ͭ��Ũ���ᷴӦ��SO2��������Ʒ����Һ��ɫ������ʲô������ж�ͭ��Ũ���ᷴӦ������ͭ���ɼ�����Һ����ɫ��д����װ���з�������Ҫ��Ӧ�Ļ�ѧ����ʽCu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O��
��2��װ���ҵ��Թܿڲ�����һ�Ž��м�Һ����������ͨ���ǽ��б���̼������Һ����������β�����գ���ֹ����������Ⱦ������
��3�����ɵ�����ʹƷ����Һ����ɫ��ɫ��ʵ����Ϻ�ȡ�������Թ�����Һ���Թ��м��ȣ�����������ɫ���ɫ��ԭ���Ƕ�������Ư��ʱ���ɲ��ȶ�����ɫ���ʣ�
���� ��1�������������Ư���ԣ�ͨ����Ʒ����Һ���������������ͭ��Һ��ʾ��ɫ���ݴ˿��ж��Ƿ�����������ͭ��ͭ��Ũ�����ڼ��������·�Ӧ��������ͭ�����������ˮ��
��2�����������ж������������ܹ���̼������Һ��Ӧ�������Ķ������������̼������Һ���գ�
��3�������������Ư���ԣ��ܹ�ʹƷ����Һ��ɫ�����������Ư����������һ�ֲ��ȶ�����ɫ���ʣ�Ϊ��ʱ�ģ����Ⱥ��ָ���
��� �⣺��1��Ũ�����ͭ�ڼ��ȵ���������������ͭ�����������ˮ�����ڶ����������Ư���ԣ���ʹ��Ʒ����Һ��ɫ���ʳ���Ʒ����Һ��֤��������Ĵ��ڣ���Ʒ����ɫ����֤���ж����������ɣ�������Һ�����ɫ��֤��������ͭ���ɣ�ͭ��Ũ���ᷴӦ��ͭ���л�ԭ�ԣ�Ũ�������ǿ�����ԣ���Ӧ������Ȳ��ܷ�������д��ѧ����ʽʱע�⡰Ũ���֣���Ӧ�Ļ�ѧ����ʽΪ��Cu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O��
�ʴ�Ϊ������Ʒ����Һ��ɫ��������Һ����ɫ��Cu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O��
��2������������һ���ж����壬���������ܹ���̼������Һ��Ӧ������װ���ҵ��Թܿڲ�����һ�Ž��б���̼������Һ�������������ն���Ķ�������ֹ��Ⱦ������
�ʴ�Ϊ��β�����գ���ֹ����������Ⱦ������
��3�����������ܹ�ʹƷ����Һ��ɫ���������ɵ�����ʹƷ����Һ����ɫ��ɫ��
���������Ư��ԭ��Ϊ��������������ɫ����������һ�ֲ��ȶ�����ɫ���ʣ����Ⱥ����ɵ���ɫ���ʷֽ⣬�ָֻ�ԭ������ɫ������ȡ�������Թ�����Һ���Թ��м��ȣ���Һ������ɫ��ɺ�ɫ��
�ʴ�Ϊ����ɫ������ɫ���ɫ����������Ư��ʱ���ɲ��ȶ�����ɫ���ʣ�
���� ���⿼����ͭ��Ũ��������ʣ���Ŀ�Ѷ��еȣ������漰ͭ��Ũ����ķ�Ӧ��������������ʼ����顢����ʵ�鷽������������۵�֪ʶ��ע����ȷ������������ˮ��Ư��ԭ��������

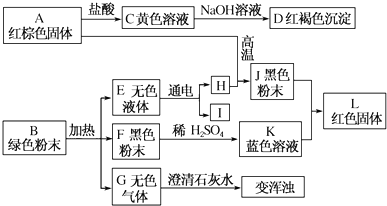
| A�� | ŨH2SO4��ϡH2SO4���ѻӷ� | |
| B�� | ŨH2SO4��ϡH2SO4���������ԣ���ŨH2SO4��ϡH2SO4���������Ե��� | |
| C�� | ŨH2SO4��ϡH2SO4�ڼ���ʱ������ͭ��Ӧ | |
| D�� | ŨH2SO4��ϡH2SO4�ڳ����¶����������������� |
 ������˵����ȷ���ǣ�������
������˵����ȷ���ǣ�������| A�� | ���� �Ľṹ�ص��֪������ϩ�ķ���ʽΪC10H8 �Ľṹ�ص��֪������ϩ�ķ���ʽΪC10H8 | |
| B�� | ���³�ѹ�»�����ϩ��һ��������ˮ����ɫ���� | |
| C�� | ������ϩ����ʹ��ˮ��ɫ������ʹ����KMnO4��Һ��ɫ | |
| D�� | ��ϩ�ͻ�����ϩ��Ϊͬϵ�� |
| Ԫ�� | �����Ϣ |
| X | X������������Ӧ��ˮ���ﻯѧʽΪH2XO3 |
| Y | Y�ǵؿ��к�����ߵ�Ԫ�� |
| Z | Z�Ļ�̬ԭ�����������Ų�ʽΪ3s23p1 |
| W | W��һ�ֺ��ص�������Ϊ28��������Ϊ14 |
��2��Z�ĵ�һ�����ܱ�W��С�����С������ XY2�ɹ�̬��Ϊ��̬����˷��������������Ƿ��Ӽ�����������Ԫ�ء�X��Y��ԭ�ӿɹ�ͬ�γɶ��ַ��ӣ�д������һ�����γ�ͬ�ַ��Ӽ��������������CH3CH2OH��CH3COOH�ȣ�
��3��W�ĵ���������ᷴӦ����������ɫ���壬�÷�Ӧ�Ļ�ѧ����ʽ��Si+4HF=SiF4��+2H2����
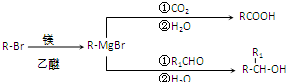
 ��
�� ��
��
 ��
��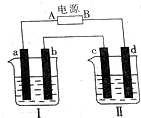 ��ͼ��ʾװ���У��ձ����зֱ�ʢ��200g 9.4%��Cu��NO3��2��Һ�������ı���K2SO3��Һ�����õ缫��Ϊ���Ե缫����ش��������⣺
��ͼ��ʾװ���У��ձ����зֱ�ʢ��200g 9.4%��Cu��NO3��2��Һ�������ı���K2SO3��Һ�����õ缫��Ϊ���Ե缫����ش��������⣺