��Ŀ����
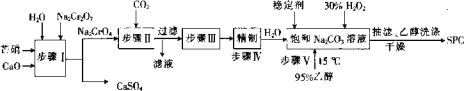
����Ŀ���ظ���س������л��ϳɵ��������ʹ����ȡ��ɺ�����Һ(��Ҫ��Cr3+��Fe3+��K+��SO42-��)�Ʊ�K2Cr2O7��������ͼ��ʾ��

��֪��i.�����������£�H2O2�ܽ�Cr2O72-��ԭΪCr3+��
ii.��ؽ��������γ��������������pH��Χ���£�
�������� | Fe3+ | Cr3+ |
��ʼ������pH | 1.5 | 4.9 |
������ȫ��pH | 2.8 | 6.8 |
�ش��������⣺
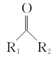
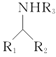
(1)�����ٵ���Ҫ�ɷ�Ϊ_________(�ѧʽ)��
(2)"�����������з�����Ӧ�����ӷ���ʽΪ____________��
(3)������"������Ŀ����_________��
(4)���ữ"�����з����ķ�ӦΪ2CrO42- +2H+![]() Cr2O72-+ H2O (K=4.0��1014L3��mol -3)����֪���ữ������Һ(pH=1)��c(Cr2O72- )=6. 4��10-3 mol��L-1������Һ��c(CrO42-)=_______��
Cr2O72-+ H2O (K=4.0��1014L3��mol -3)����֪���ữ������Һ(pH=1)��c(Cr2O72- )=6. 4��10-3 mol��L-1������Һ��c(CrO42-)=_______��
(5)�����������ؽ��Ƹ���ˮ�е�Cr2O72-����ԭ���乤��ԭ����ͼ��ʾ��
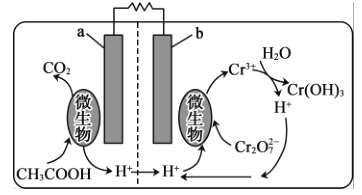
�ڸõ���У�b��______����a���ĵ缫��ӦΪ__________
(6)K2 Cr2O7����Һ�������ڲⶨ������������ƾ���(Na2S2O3��5H2O��M=248 g��mol -1)�Ĵ��ȡ��ⶨ�������£�
i.��Һ���ƣ���ȡ1.2400g������������ƾ�����Ʒ��������в���ȴ������ˮ���ձ����ܽ⣬��ȫ�ܽ��.ȫ��ת����100mL����ƿ��.������ˮ���̶��ߡ�
ii.�ζ���ȡ0.01000 mol. L-1��K2Cr2 O7����Һ20. 00 mL��ϡ�����ữ��������KI��Һ��������Ӧ(Cr2O72-����ԭ��Cr3+ ��I-��������I2)��Ȼ���������������Ʒ��Һ�ζ���������ɫ��������Ӧ��I2+2S2O32 - =S4O62-+2I-�����������Һ��Ϊָʾ���������ζ�������Һ��ɫ��ȥ.��Ϊ�յ㡣ƽ�еζ�3�Σ���Ʒ��Һ��ƽ������Ϊ25.00mL��
��ϡ�����ữ��K2Cr2O7����Һ��KI��Һ��Ӧ�����ӷ���ʽΪ_________
�ڸ�������������ƾ�����Ʒ�Ĵ���Ϊ_______%(����1λС��)��
���𰸡�Cr(OH)3��Fe(OH)3 2Cr(OH)3+3H2O2+4OH-=2CrO42-+8H2O ��ȥ������H2O2 ![]() �� CH3COOH8e+2H2O=2CO2��+8H+ Cr2O72-+14H++6I-=3I2+2Cr3++7H2O 96.0
�� CH3COOH8e+2H2O=2CO2��+8H+ Cr2O72-+14H++6I-=3I2+2Cr3++7H2O 96.0
��������
������Һ����Ҫ��Cr3+��Fe3+��K+��SO42-�ȣ��Ʊ�K2Cr2O7�����̣���Һ������KOH��Ӧ���ɳ���Cr��OH��3��Fe��OH��3�����˵õ�������Cr��OH��3��Fe��OH��3������ȥK+��SO42-�����ӣ������������������м��������������Cr��OH��3����K2CrO4�����˳�ȥFe��OH��3���õ�K2CrO4��Һ�ڣ����ȳ�ȥ�����������⣬��ֹ��������ʱH2O2�ܽ�Cr2O72-��ԭΪCr3+�������pH=1��ʹK2CrO4��Һת��ΪK2Cr2O7��Һ�������ᾧ�õ�K2Cr2O7���壬�Դ˽��
��1�������γ��������������pH��Χ֪�����ٳɷ�ΪCr��OH��3��Fe��OH��3�����ʴ�Ϊ��Cr(OH)3��Fe(OH)3��
��2���������������У����������£�������������Cr(OH)3������K2Cr2O7��Һ�����ת�Ƶ����غ㡢ԭ���غ�û�ѧ����ʽΪ2Cr(OH)3+3H2O2+4KOH=2K2CrO4+8H2O�������ӷ���ʽΪ��2Cr(OH)3+3H202+4OH-=2CrO42-+8H20���ʴ�Ϊ��2Cr(OH)3+3H2O2+4OH-=2CrO42-+8H2O��
��3����Ϊ�����������£�H2O2�ܽ�Cr2O72-��ԭΪCr3+��Ϊ��ֹ�������ữ��������Cr2O72-��Cr2O72-��ԭΪCr3+���������ʣ������ữ֮ǰ�����ȥH2O2������H2O2���Ȳ��ȶ����ֽ⣬ͨ�����ȿɳ�ȥ���ʴ�Ϊ����ȥ������H2O2��
��4�����ữ������Һ(pH=1)����c(H+)=0.1mol/L����Ӧ2CrO42- +2H+![]() Cr2O72-+ H2O K=
Cr2O72-+ H2O K=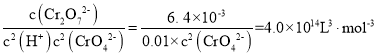 ����
����![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��5��b����Cr2O72����ԭ��ӦΪ������Ӧ����aΪ���������ᱻ�������ɶ�����̼���缫����ʽΪ��CH3COOH8e+2H2O=2CO2��+8H+���ʴ�Ϊ������CH3COOH8e+2H2O=2CO2��+8H+��
��6���������������£�Cr2O72-����ԭ��Cr3+ ��I-��������I2��ͬʱ��ˮ���ɣ����ݵ���ת���غ���ƽ��Ӧ�ã�Cr2O72-+14H++6I-=3I2+2Cr3++7H2O���ʴ�Ϊ��Cr2O72-+14H++6I-=3I2span>+2Cr3++7H2O��
�ڸ���������ԭ��Ӧ�е���ת���غ�ã�n(I2)=3n(Cr2O72-)=3��0.01000 mol. L-1��0.02L=0.0006mol������I2+2S2O32 - =S4O62-+2I-�ã�n(Na2S2O3)=2n(I2)= 0.0006mol��2=0.0012mol�����������������ƾ�����Ʒ�Ĵ���Ϊ ���ʴ�Ϊ��96.0%��
���ʴ�Ϊ��96.0%��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ�����¶�t1��t2�£�X2(g)��H2��Ӧ����HX��ƽ�ⳣ�����±�
��ѧ����ʽ | K(t1) | K(t2) |
F2��H2 | 1.8��1036 | 1.9��1032 |
Cl2��H2 | 9.7��1012 | 4.2��1011 |
Br2��H2 | 5.6��107 | 9.3��106 |
I2��H2 | 43 | 34 |
����K�ı仯���������֪ʶ�Ʋ⣬����±��ԭ�Ӻ˵�����Ľ��ͣ�����˵������ȷ����
A.����ͬ�����£�ƽ��ʱX2��ת����������
B.X2��H2��Ӧ�ľ��ҳ̶�����
C.HX�Ļ�ԭ������
D.HX������Ϊ���ȷ�Ӧ���ɴ˿�֪t1<t2