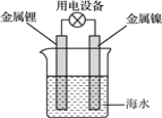
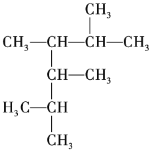
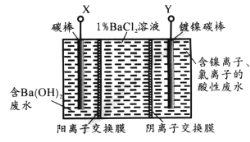
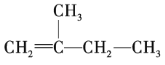ЬтФПФкШн
ЁОЬтФПЁПЯђфхЫЎжаМгШызуСПЕФввШЉШмвКЃЌПЩвдПДЕНфхЫЎЭЪЩЋЃЌЖдВњЩњИУЯжЯѓЕФдвђгаШчЯТШ§жжВТЯыЃКЂйфхЫЎгыввШЉЗЂЩњШЁДњЗДгІЃЛЂкгЩгкввШЉЗжзгжагаВЛБЅКЭМќЃЌфхЫЎгыввШЉЗЂЩњМгГЩЗДгІЃЛЂлгЩгкввШЉОпгаЛЙдадЃЌфхЫЎНЋввШЉбѕЛЏЮЊввЫсЁЃЮЊЬНОПФФжжВТЯые§ШЗЃЌвЛбаОПадбЇЯАаЁзщЬсГіСЫШчЯТСНжжЪЕбщЗНАИЃК
ЗНАИвЛЃКМьбщЭЪЩЋКѓШмвКЕФЫсМюадЃЛ
ЗНАИЖўЃКВтЖЈЗДгІЧАфхЫЎжа Br2 ЕФЮяжЪЕФСПКЭЗДгІКѓШмвКжа BrЁЊРызгЕФЮяжЪЕФСПЁЃ
ЃЈ1ЃЉЗНАИвЛЪЧЗёПЩаа__________Ью(ЁАЪЧЁБЛђЁАЗёЁБЃЉЃЌРэгЩЪЧ____________________ЁЃ
ЃЈ2ЃЉМйЩшВтЕУЗДгІЧАфхЫЎжа Br2 ЕФЮяжЪЕФСПЮЊ amolЃЌ
ШєВтЕУЗДгІКѓ n(BrЃЃЉЃН__________molЃЌдђЫЕУїфхЫЎгыввШЉЗЂЩњШЁДњЗДгІЃЛ
ШєВтЕУЗДгІКѓ n(BrЃЃЉЃН__________molЃЌдђЫЕУїфхЫЎгыввШЉЗЂЩњМгГЩЗДгІЃЛ
ШєВтЕУЗДгІКѓ n(BrЃЃЉЃН__________molЃЌдђЫЕУїфхЫЎНЋввШЉбѕЛЏЮЊввЫсЁЃ
ЃЈ3ЃЉАДЮяжЪЕФСПжЎБШЮЊ 1ЉU5 ХфжЦ 1000mLKBrO3ЃKBr ШмвКЃЌИУШмвКдкЫсадЬѕМўЯТЭъШЋЗДгІПЩЩњГЩ 0.5molBr2ЁЃШЁИУШмвК 10mL МгШызуСПввШЉШмвКЃЌЪЙЦфЭЪЩЋЃЌШЛКѓНЋЫљЕУШмвКЯЁЪЭЮЊ 100mLЃЌзМШЗСПШЁЦфжа 10mLЃЌМгШыЙ§СПЕФ AgNO3 ШмвКЃЌЙ§ТЫЁЂЯДЕгЁЂИЩдяКѓГЦСПЕУЕНЙЬЬх 0.188gЁЃШєвбжЊ CH3COOAg взШмгкЫЎЃЌЪдЭЈЙ§МЦЫуХаЖЯфхЫЎгыввШЉЗЂЩњЗДгІЕФРраЭЮЊ__________(бЁЬюВТЯыађКХЃЉЁЃ
ЃЈ4ЃЉаДГіЩЯЪіВтЖЈЙ§ГЬжаЕФШ§ИіЗДгІЕФРызгЗНГЬЪНЃК
ЂйKBrO3 КЭKBr дкЫсадЬѕМўЯТЕФЗДгІЃК______________________________ЃЛ
ЂкфхЫЎгыввШЉЕФЗДгІЃК______________________________ЃЛ
ЂлВтЖЈ BrЃРызгКЌСПЕФЗДгІЃК______________________________ЃЛ
ЁОД№АИЁП
ЃЈ1ЃЉЗёЃЌфхЫЎжаКЌгаHBrЃЌШмвКБОЩэГЪЫсадЃЛ
ЃЈ2ЃЉaЁЂ0ЁЂ2aЃЛ
ЃЈ3ЃЉЂл
ЃЈ4ЃЉЂйBrO3-+5Br-+6H+=3Br2+3H2OЃЛЂкCH3CHO+Br2+H2O=CH3COOH+2H++2Br-ЃЛЂл![]()
ЁОНтЮіЁП
ЪдЬтЗжЮіЃКЃЈ1ЃЉвђфхЫЎгыввШЉЗЂЩњШЁДњЗДгІгаHBrЩњГЩЃЛввШЉБЛбѕЛЏЩњГЩввЫсКЭHBrЃЌШмвКОљГЪЫсадЃЌЙЪВЛФмгУВтЖЈШмвКЫсМюадЕФЗНЗЈЃЌЗНАИвЛВЛПЩааЃЌЙЪД№АИЮЊЃКВЛПЩааЃЛфхЫЎгыввШЉЗЂЩњШЁДњЗДгІКЭввШЉБЛфхЫЎбѕЛЏЖМгафхЛЏЧтЩњГЩЃЌШмвКОљЯдЫсадЃЛ
ЃЈ2ЃЉШєЗЂЩњШЁДњЗДгІЃЌ1molBr2ПЩЩњГЩ1molHBrЃЌЙЪЗДгІКѓn(Br-ЃЉ=amolЃЛШєЗЂЩњМгГЩЗДгІЃЌдђЮоHBrЩњГЩЃЌЙЪЗДгІКѓn(Br-ЃЉ=0ЃЛШєЗЂЩњбѕЛЏЗДгІЃЌ1molBr2ПЩБЛЛЙдЩњГЩ2molHBrЃЌЙЪЗДгІКѓn(Br-ЃЉ=2amolЃЌЙЪД№АИЮЊЃКaЃЛ0ЃЛ2aЃЛ
ЃЈ3ЃЉгыввШЉЗДгІЕФBr2ЮЊ0.5ЁС![]() =0.005molЃКn(AgBrЃЉ=
=0.005molЃКn(AgBrЃЉ=![]() =0.001molЃЌЙЪBr2гыввШЉЗДгІЩњГЩЕФBr-ЮЊЃК0.001molЁС
=0.001molЃЌЙЪBr2гыввШЉЗДгІЩњГЩЕФBr-ЮЊЃК0.001molЁС![]() =0.01molЃЌЙЪn(Br-ЃЉЃКn(Br2ЃЉ=0.01ЃК0.005=2ЃК1ЃЌЙЪЗЂЩњбѕЛЏЗДгІЃЌЙЪД№АИЮЊЃКЂлЃЛ
=0.01molЃЌЙЪn(Br-ЃЉЃКn(Br2ЃЉ=0.01ЃК0.005=2ЃК1ЃЌЙЪЗЂЩњбѕЛЏЗДгІЃЌЙЪД№АИЮЊЃКЂлЃЛ
ЃЈ4ЃЉЂйKBrO3гыKBrдкЫсадЬѕМўЯТЗЂЩњбѕЛЏЛЙдЗДгІЃЌЩњГЩЕЅжЪфхЃЌЗНГЬЪНЮЊЃКBrO3-+5Br-+6H+=3Br2+3H2OЃЌЙЪД№АИЮЊЃКBrO3-+5Br-+6H+=3Br2+3H2OЃЛ
ЂкфхЫЎгыввШЉЗЂЩњбѕЛЏЛЙдЗДгІЃЌЗНГЬЪНЮЊЃКCH3CHO+Br2+H2O=CH3COOH+2H++2Br-ЃЌЙЪД№АИЮЊЃКCH3CHO+Br2+H2O=CH3COOH+2H++2Br-ЃЛ
ЂлВтЖЈ BrЃРызгКЌСПжаЗЂЩњЕФЗДгІЮЊ![]() ЃЌЙЪД№АИЮЊЃК
ЃЌЙЪД№АИЮЊЃК![]() ЁЃ
ЁЃ

ЁОЬтФПЁПЛњЖЏГЕХХЗХЕФЮлШОЮяжївЊгаЬМЧтЛЏКЯЮяЁЂвЛбѕЛЏЬМКЭЕЊбѕЛЏЮяЕШЁЃ
IЃЎЦћгЭШМгЭГЕЩЯАВзАШ§дЊДпЛЏзЊЛЏЦїЃЌПЩгааЇНЕЕЭЦћГЕЮВЦјЮлШОЁЃ
(1)вбжЊЃК C(s)+O2(g) = CO2(g) ЁїH1 ЃН 393.5kJЁЄmol1
2C(s)+O2(g) = 2CO(g) ЁїH2 ЃН 221.0 kJЁЄmol1
N2(g)+O2(g) = 2NO(g) ЁїH 3 ЃН+180.5 kJЁЄmol1
COКЭNOСНжжЮВЦјдкДпЛЏМСзїгУЯТЩњГЩN2ЕФШШЛЏбЇЗНГЬЪНЪЧ_________________________ЁЃ
(2)баОПCOКЭNOЕФДпЛЏЗДгІЃЌгУЦјЬхДЋИаЦїВтЕУдкФГЮТЖШЯТЁЂвЛЖЈЬхЛ§ЕФУмБеШнЦїжаЃЌВЛЭЌЪБМфNOКЭCOХЈЖШШчЯТБэЃК
ЪБМфЃЈsЃЉ | 0 | 1 | 2 | 3 | 4 | 5 |
c(NO)/ЃЈ______ЃЉ4molЁЄL1) | 10.0 | 4.50 | 2.50 | 1.50 | 1.00 | 1.00 |
c(CO)/ЃЈ______ЃЉ3molЁЄL1) | 3.60 | 3.05 | 2.85 | 2.75 | 2.70 | 2.70 |
Ђй ЧА4 sФкЕФЦНОљЗДгІЫйТЪІд(CO) ЃН______molЁЄL1ЁЄs1ЁЃ
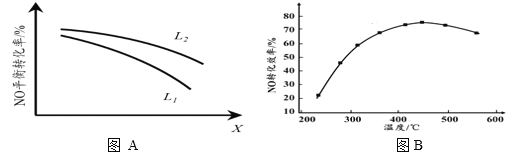
Ђк LЁЂXПЩЗжБ№ДњБэбЙЧПЛђЮТЖШЁЃЯТЭМAБэЪОLвЛЖЈЪБЃЌNO(g)ЕФЦНКтзЊЛЏТЪЫцXЕФБфЛЏЙиЯЕЁЃXДњБэЕФЮяРэСПЪЧ______ЁЃХаЖЯL1ЁЂL2ЕФДѓаЁЙиЯЕЃЌВЂМђЪіРэгЩЃК______________________________ЁЃ
(3)ЪЕбщВтЕУЃЌvе§=kе§ЁЄc2(NO)ЁЄc2(CO)ЃЌvФц=kФцЁЄc(N2) ЁЄc2(CO2)ЃЈkе§ЁЂkФцЮЊЫйТЪГЃЪ§ЃЌжЛгыЮТЖШгаЙиЃЉЁЃ
ЂйДяЕНЦНКтКѓЃЌНіЩ§ИпЮТЖШЃЌkе§діДѓЕФБЖЪ§______ЃЈЬюЁА>ЁБЁЂЁА
ЂкШєдк2 LЕФУмБеШнЦїжаГфШы1 mol COКЭ1 mol NOЃЌдквЛЖЈЮТЖШЯТДяЕНЦНКтЪБЃЌCOЕФзЊЛЏТЪЮЊ40%ЃЌдђkе§ЉUkФц =___________ЁЃЃЈБЃСєвЛЮЛаЁЪ§ЃЉ
II. гаШЫРћгУЗДгІC(s)+2NO(g) ![]() N2(g)+CO2(g) ІЄH = 34.0 kJЁЄmol1ЃЌгУЛюадЬПЖдNOНјааЮќИНЁЃЯждкУмБеШнЦїжаМгШызуСПЕФCКЭвЛЖЈСПЕФNOЦјЬхВЂдкДпЛЏМСзїгУЯТЗЂЩњЗДгІЃЌОЯрЭЌЪБМфВтЕУNOЕФзЊЛЏТЪЫцЮТЖШЕФБфЛЏШчЭМBЫљЪОЁЃгЩЭМПЩжЊзюИпзЊЛЏТЪЖдгІЮТЖШЮЊ450ЁцЁЃЕЭгк450ЁцЪБЃЌNOЕФзЊЛЏТЪЪЧВЛЪЧЖдгІЮТЖШЯТЕФЦНКтзЊЛЏТЪМАХаЖЯРэгЩЪЧ________________________ЃЛ
N2(g)+CO2(g) ІЄH = 34.0 kJЁЄmol1ЃЌгУЛюадЬПЖдNOНјааЮќИНЁЃЯждкУмБеШнЦїжаМгШызуСПЕФCКЭвЛЖЈСПЕФNOЦјЬхВЂдкДпЛЏМСзїгУЯТЗЂЩњЗДгІЃЌОЯрЭЌЪБМфВтЕУNOЕФзЊЛЏТЪЫцЮТЖШЕФБфЛЏШчЭМBЫљЪОЁЃгЩЭМПЩжЊзюИпзЊЛЏТЪЖдгІЮТЖШЮЊ450ЁцЁЃЕЭгк450ЁцЪБЃЌNOЕФзЊЛЏТЪЪЧВЛЪЧЖдгІЮТЖШЯТЕФЦНКтзЊЛЏТЪМАХаЖЯРэгЩЪЧ________________________ЃЛ
ЁОЬтФПЁПЃЈ1ЃЉЯТСа3жжВЛЭЌСЃзг![]() HЁЂ
HЁЂ![]() HЁЂ
HЁЂ![]() HБэЪО______жждЊЫиЃЌ______жжКЫЫиЃЌ
HБэЪО______жждЊЫиЃЌ______жжКЫЫиЃЌ![]() HЁЂ
HЁЂ![]() HЁЂ
HЁЂ![]() HЛЅГЦЮЊ__________________ЁЃ
HЛЅГЦЮЊ__________________ЁЃ
ЃЈ2ЃЉгаШЫГЦУКЬПЪЧЁАЙЄвЕЕФСИЪГЁБЃЌЭЈЙ§УКЕФзлКЯРћгУПЩвдЛёЕУживЊЛЏЙЄдСЯЃЌШчНЙЬПЃЌЫќЪЧУКЕФ______ (ЬюЁАеєСѓЁБЛђЁАИЩСѓЁБ)ВњЮяЃЌПЩгУгк________________ (ЬюГівЛжжгУЭО)ЃЛвдУКЮЊдСЯПЩвджЦЕУЫЎУКЦјЃЌЦфЗДгІЕФЛЏбЇЗНГЬЪНЮЊ________________________________ЁЃ
ЃЈ3ЃЉдЊЫиЕФЕчИКадЕФДѓаЁПЩвдзїЮЊХаЖЯдЊЫиН№ЪєадгыЗЧН№ЪєадЧПШѕЕФГпЖШЁЃЯТБэСаГіВПЗжЖЬжмЦкдЊЫиЕФЕчИКадЁЃЧыЛиД№ЯТСаЮЪЬтЁЃ
дЊЫи | Al | B | Be | C | Cl | X | Li |
ЕчИКад | 1.5 | 2.0 | 1.5 | 2.5 | 3.0 | 4.0 | 1.0 |
дЊЫи | Mg | N | Y | O | P | S | Si |
ЕчИКад | 1.2 | 3.0 | 0.9 | 3.5 | 2.1 | 2.5 | 1.8 |
ЂйИљОнЩЯБэИјГіЕФЪ§ОнЃЌПЩжЊдЊЫиЕФЕчИКаддНДѓЃЌ____________(ЬюЁАН№ЪєадЁБЛђЁАЗЧН№ЪєадЁБ)дНЧПЁЃ
ЂкЭЦВтY ЮЊ______(ЬюдЊЫиЗћКХ)ЃЌгУЕчзгЪНБэЪОXдЊЫигыYдЊЫиЕФЛЏКЯЮяЕФаЮГЩЙ§ГЬ__________________ЁЃ