��Ŀ����
2011��3��26�գ�����ʡ�����������з����˼������˹������Ժ��ص�-131��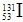 ��������ȷ��ϵ�����ձ��˵�վ�¹ʷ��������ʵ��ͷţ�����Щ�����ķ��������ʶ��ҹ��������ڽ����������Ӱ�죬����غ�����Ҳ�����Ʒ��֡������й�˵����ȷ����
��������ȷ��ϵ�����ձ��˵�վ�¹ʷ��������ʵ��ͷţ�����Щ�����ķ��������ʶ��ҹ��������ڽ����������Ӱ�죬����غ�����Ҳ�����Ʒ��֡������й�˵����ȷ����
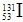 ��������ȷ��ϵ�����ձ��˵�վ�¹ʷ��������ʵ��ͷţ�����Щ�����ķ��������ʶ��ҹ��������ڽ����������Ӱ�죬����غ�����Ҳ�����Ʒ��֡������й�˵����ȷ����
��������ȷ��ϵ�����ձ��˵�վ�¹ʷ��������ʵ��ͷţ�����Щ�����ķ��������ʶ��ҹ��������ڽ����������Ӱ�죬����غ�����Ҳ�����Ʒ��֡������й�˵����ȷ����| A����-131��Ԫ�ص��һ��ͬλ�أ������������Ȼ���Dz������ |
| B����-131�Ļ�ѧ������Ԫ�صⲻͬ |
| C����-131��ԭ�Ӻ�����74������ |
| D����Ԫ�ص����ԭ�������ǰ��հ�����-131���ڸ��ֺ���ԭ����ռ��һ���ٷֱ������ƽ��ֵ |
A
A��ȷ����-131���˹������Ժ��أ���Ԫ�ص��һ��ͬλ�أ������������Ȼ���Dz�����ڣ�
B����ͬλ��ԭ�ӵĻ�ѧ������ͬ�����ƣ�
C����.��-131��ԭ�Ӻ�����78�����ӣ��ɸ���������=��������������=131��53=78��
D����Ԫ�ص����ԭ��������ָ��������Ȼͬλ��ԭ����ռ��һ���ٷֱ��������ƽ��ֵ��
B����ͬλ��ԭ�ӵĻ�ѧ������ͬ�����ƣ�
C����.��-131��ԭ�Ӻ�����78�����ӣ��ɸ���������=��������������=131��53=78��
D����Ԫ�ص����ԭ��������ָ��������Ȼͬλ��ԭ����ռ��һ���ٷֱ��������ƽ��ֵ��

��ϰ��ϵ�д�
�����Ŀ
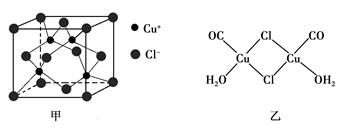
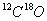 ��
��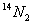 �������壬���������������������˵����ȷ����
�������壬���������������������˵����ȷ����