��Ŀ����
��ͼ��Ԫ�����ڱ���һ����
��1��д��Ԫ�آ�Ļ�̬ԭ�ӵļ۵����Ų�ʽ_______ ______��ָ���������ڱ��е�λ��_______________�����Ӹֹ�ʱ�������â��ijЩ��������ߵĵ����ڸ����·�����Ӧ����д������һ�ַ�Ӧ�Ļ�ѧ����ʽ__ ��
��2���٢ۢ�����Ԫ�ؿ����γɶ����л���������ӣ��������ԭ�������ٵ�һ��������װ��ʱ�γɵ���Ҫ������Ⱦ���д�����ĵ���ʽ_ _�����ݼ۲���ӶԻ��⣨VSEPR�������Ʋ�÷��ӵĿռ乹��Ϊ__ ��
��3���ۢܢݢޢ�����Ԫ�ض�������Ԫ�آ��γɻ���������۵���ߵ���________(д������Ļ�ѧʽ)��������¶Ƚӽ�373Kʱ������M=m/n�ⶨ�ݵ���̬�⻯�����Է���������������ֲⶨ���������ֵ�ߣ���ԭ���� ��
��4��ijЩ��ͬ��Ԫ�ص�����Ҳ��һ���������ԣ����ϱ���Ԫ�آ���Ԫ�آڵ��������������Ƶ����ʡ���д��Ԫ�آڵ�����������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��
��
| �� | | | | | | | | | | | | | | | | | |
| | �� | | | | | | | | | | | | �� | �� | �� | | |
| �� | | | | | | | | | | | | �� | | | | �� | |
| | | | �� | | | | �� | | | | | | | | | | |
��2���٢ۢ�����Ԫ�ؿ����γɶ����л���������ӣ��������ԭ�������ٵ�һ��������װ��ʱ�γɵ���Ҫ������Ⱦ���д�����ĵ���ʽ_ _�����ݼ۲���ӶԻ��⣨VSEPR�������Ʋ�÷��ӵĿռ乹��Ϊ__ ��
��3���ۢܢݢޢ�����Ԫ�ض�������Ԫ�آ��γɻ���������۵���ߵ���________(д������Ļ�ѧʽ)��������¶Ƚӽ�373Kʱ������M=m/n�ⶨ�ݵ���̬�⻯�����Է���������������ֲⶨ���������ֵ�ߣ���ԭ���� ��
��4��ijЩ��ͬ��Ԫ�ص�����Ҳ��һ���������ԣ����ϱ���Ԫ�آ���Ԫ�آڵ��������������Ƶ����ʡ���д��Ԫ�آڵ�����������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��
��
��1��ls22s22p63s23p63d64s2 �������ڢ��� ���ȼ���Ӧʽ
��2��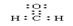 ƽ��������
ƽ��������
��3��NaH �ӽ��е��ˮ�����д����൱����ͨ��������γɵĵϷ���
 ��4��Be(OH)2+2NaOH=Na2BeO2+2H2O
��4��Be(OH)2+2NaOH=Na2BeO2+2H2O
��2��
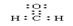 ƽ��������
ƽ����������3��NaH �ӽ��е��ˮ�����д����൱����ͨ��������γɵĵϷ���
 ��4��Be(OH)2+2NaOH=Na2BeO2+2H2O
��4��Be(OH)2+2NaOH=Na2BeO2+2H2O��

��ϰ��ϵ�д�
 ��ĩ100�ִ��غ�������ϵ�д�
��ĩ100�ִ��غ�������ϵ�д� Сѧ�������Ծ�ϵ�д�
Сѧ�������Ծ�ϵ�д�
�����Ŀ
 ��������ȷ��ϵ�����ձ��˵�վ�¹ʷ��������ʵ��ͷţ�����Щ�����ķ��������ʶ��ҹ��������ڽ����������Ӱ�죬����غ�����Ҳ�����Ʒ��֡������й�˵����ȷ����
��������ȷ��ϵ�����ձ��˵�վ�¹ʷ��������ʵ��ͷţ�����Щ�����ķ��������ʶ��ҹ��������ڽ����������Ӱ�죬����غ�����Ҳ�����Ʒ��֡������й�˵����ȷ����