��Ŀ����
����Ŀ��ijʵ��С�������̿��Ʊ�������أ���Ҫʵ�鲽�����£�
����һ�������̿�(��Ҫ�ɷ�ΪMnO2)��KClO3��KOH���尴һ��������Ϻ�������ڿ��Ƶ���ɫK2MnO4��
�����������Ӧ��������ܽ⡢���ˣ�����Һ��ͨ�����CO2����ʹ��Һ�����ԣ�K2MnO4�����������·�Ӧ�õ�KMnO4��MnO2��K2CO3��������õ�������ؾ���(�ֲ�Ʒ)��
��ش��������⣺
(1)����һ��������Ӧ��________(�����)���С�
A.������ B.���������� C.������ D.ʯӢ
(2)Ϊ��һ����ߴֲ�Ʒ(������ؾ���)�Ĵ��ȣ������õ�ʵ�鷽����_____________��
(3)KMnO4��MnO2��һ�������¶���������Ũ������ȡ��������ȡ�����ʵ�������������KMnO4��MnO2�����ʵ���֮��Ϊ________________��
(4)ijѧϰС��Ϊ��̽�����������Һ�Ͳ�������Һ�ķ�Ӧ���̣������������Һ��ε���һ����������Բ�������Һ��(�¶���ͬ����������)����¼�����������
����KMnO4��Һ�Ĵ���(����ÿ����Һ������) | �����KMnO4��Һ��ɫ��ȥ��ʱ�� |
�ȵ����1�� | 1min |
��ɫ���ٵ����2�� | 15s |
��ɫ���ٵ����3�� | 3s |
��ɫ���ٵ����4�� | 1s |
��������������Һ��ɫʱ��仯��ԭ��_________��
(5)�ҹ������ڿ������������涨���ڿ����м�ȩ(HCHO)�������ó���0.08mg/m3����Уijͬѧͨ���������ϣ���������·����ⶨ2019�������װ�Ļ�Ȼһ�µ�����¥�����ڿ����м�ȩ�ĺ�����
�ⶨԭ����5HCHO+4KMnO4+6H2SO4=2K2SO4+4MnSO4+5CO2��+11H2O��
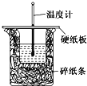
ʵ����������ݻ�Ϊ1000mL��ע������ȡ����������ע�����Ը��������Һ��(װ����ͼ)�������Ը��������Һ������ɫʱ��ֹͣע�롣

����0.1000mol/L������KMnO4��Һ����ʵ����ʹ�õ�����KMnO4��Һl000mL����0.1000mol/L������KMnO4��Һ�����Ϊ___________��
��װ���ж�ײ������ݵ�������__________��
�۸�ͬѧ��206�����ڲ�ͬ�ص��ȡ������������ע�����Ը��������Һ�С�����250�ν�ע�����п���ȫ������ע�����Ը��������Һ��ʱ�����Ը��������Һǡ����ɫ����ͨ�������жϣ��ý��ҿ����м�ȩ����________(��ѡ���δ��)���ꡣ��δ���꣬�ý����ڿ����м�ȩ(HCHO)����Ϊ________mg/m3�����ѳ��꣬��ٳ��������ڿ�����Ⱦ��һ��������ʩ��_________________��
���𰸡�A �ؽᾧ 2:5 ��Ӧ���ɵ�Mn2+�Է�Ӧ�д����ã���c(Mn2+)Խ���Ч��Խ�� 1.00mL ʹ��ȩ�����Ը��������Һ������� δ 0.075
��������
(1)�����̿�(��Ҫ�ɷ�ΪMnO2)��KClO3��KOH������ȣ�ֻ������������KOH��Ӧ����ѡA��
(2) �ᴿ������ع��壬�����������ʵĺ����ɲ����ؽᾧ�ķ�����
(3)KMnO4��ȡ����ʱ�����ϼ���+7��Ϊ+2��ת��5�����ӣ�MnO2��ȡ����ʱ�����ϼ���+4��Ϊ+2��ת��2�����ӣ�����ݵ��ӵ�ʧ�غ��֪���ʵ���֮��Ϊ2��5��
(4)����ĸ������Խ�࣬��ɫʱ��Խ�̣�����Ӧ����Խ�죬˵����ԭ����Mn2+�Է�Ӧ�д��ã���c(Mn2+)Խ���Ч��Խ�á�
(5)����0.1000mol/L������KMnO4��Һ����0.0001mol/L����KMnO4��Һ1000mL��Ũ��ϡ��Ϊԭ����1000��֮һ������0.1000mol/L������KMnO4��Һ�����Ϊ1mL��
��װ���ж�ײ�����ĽӴ��������������ʹ��ȩ�����Ը��������Һ������ա�
��ʵ��ʱ�ھ����ڲ�ͬ�ص��ȡ������������ע�����Ը��������Һ�С�����250�ν�ע��������ȫ������ע�����Ը��������Һ��ʱ�����ȡ�Ŀ������Ϊ1000mL��250=0.25m3����þ������ڿ����м�ȩ����Ϊxmg/m3�����ݷ�Ӧ��ϵʽ��
![]()
![]() ��
��![]() ��5��4��x=0.075��������װ�ľ��ҿ����м�ȩ����Ϊ0.075 mg/m3��0.08 mg/m3��δ�������ҹ涨����
��5��4��x=0.075��������װ�ľ��ҿ����м�ȩ����Ϊ0.075 mg/m3��0.08 mg/m3��δ�������ҹ涨����