��Ŀ����
����Ŀ���ʻ���(COS)����������ˮ�ڴ��������µķ�Ӧ���£�
��COS(g)+H2(g)![]() H2S(g)+CO(g) ��H1=-17kJ/mol��
H2S(g)+CO(g) ��H1=-17kJ/mol��
��COS(g)+H2O(g)![]() H2S(g)+CO2(g) ��H2=-35kJ/mol��
H2S(g)+CO2(g) ��H2=-35kJ/mol��
�ش��������⣺
(1)������Ӧ������ѧ�����ƾ�������ԭ���ǣ�________________��
(2)��ӦCO(g)+H2O(g)![]() H2(g)+CO2(g)����H=_______��
H2(g)+CO2(g)����H=_______��
(3)�ʻ���������ˮ����������ϵ��ʼͶ�ϱȲ��䣬����ʻ�����ˮ������Ӧ��ѡ���ԵĹؼ�������______��
(4)�ڳ��д����ĺ�ѹ�ܱ�������ֻ���з�Ӧ��![]() ����ʼ�����n(H2)��n(COS)=m����ͬʱ���ڲ��COSת������m���¶�(T)�Ĺ�ϵ��ͼ��ʾ��
����ʼ�����n(H2)��n(COS)=m����ͬʱ���ڲ��COSת������m���¶�(T)�Ĺ�ϵ��ͼ��ʾ��

��m1______m2(����![]() ������
������![]() ������
������![]() ��
��![]() ��
��
���¶ȸ���T0��COSת���ʼ�С�Ŀ���ԭ��Ϊ��i�и�Ӧ������ii______��iii______��
(5)�ڳ��д����ĺ�ѹ�ܱ������н��з�Ӧ��.COS(g)��H2O(g)Ͷ�ϱȷֱ�Ϊ1��3��1��1����Ӧ��������ʵ�����ͬʱ��COS(g)��ƽ��ת�������¶ȵĹ�ϵ������ͼ��ʾ��
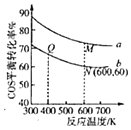
��M���Ӧ��ƽ�ⳣ��______Q��![]() ����
����![]() ������
������![]() ������
������![]() ��
��![]() ��
��
��N���Ӧ��ƽ��������COS(g)���ʵ�������Ϊ______��
��M���Q���Ӧ��ƽ��������������ʵ���֮��Ϊ______��
���𰸡�������Ӧ��Ϊ���Ƚ��ٵķ�Ӧ -18kJ/mol ѡ���Ч�Ĵ��� ![]() ����������� ƽ��������� < 20% 1��1
����������� ƽ��������� < 20% 1��1
��������
(1)�����Ȼ�ѧ����ʽ�����֪��������Ӧ���������ٵķ�Ӧ������Ӧ����������������С���������ѧ����С��
(2) ��COS(g)+H2(g)![]() H2S(g)+CO(g) ��H1=-17kJ/mol��
H2S(g)+CO(g) ��H1=-17kJ/mol��
��COS(g)+H2O(g)![]() H2S(g)+CO2(g) ��H2=-35kJ/mol��
H2S(g)+CO2(g) ��H2=-35kJ/mol��
��˹���ɼ����-��õ���CO(g)+H2O(g)![]() H2(g)+CO2(g)����H��
H2(g)+CO2(g)����H��
(3)����ʻ�����ˮ������Ӧ��ѡ���ԵĹؼ������Ǹ�Ч������
(4)���ڳ��д����ĺ�ѹ�ܱ�������ֻ���з�Ӧ������ʼ�����n(H2)��n(COS)=m��mԽ��˵��������Խ�࣬���ַ�Ӧ������һ�ֻ������һ�ֵ�ת���ʣ�
���¶ȸ���T0��COSת���ʼ�С����Ϊ�¶����ߣ��������Լ�������Ӧ������ƽ��������У�COS��ת���ʼ�С��
(5)�ٷ�Ӧ��ƽ�ⳣ�����¶ȱ仯������ƽ��������У�
��N��COSת����Ϊ60%��COS(g)��H2O(g)Ͷ�ϱ�1��1��������м�����ʽ����N���Ӧ��ƽ��������COS(g)���ʵ���������
��M��COSת����Ϊ60%��COS(g)��H2O(g)Ͷ�ϱ�1��3��N��COSת����Ϊ60%��COS(g)��H2O(g)Ͷ�ϱ�1��1����Ӧǰ���������ʵ���������㡣
(1)��COS(g)+H2(g)![]() H2S(g)+CO(g) ��H1=-17kJ/mol��
H2S(g)+CO(g) ��H1=-17kJ/mol��
��COS(g)+H2O(g)![]() H2S(g)+CO2(g) ��H2=-35kJ/mol��
H2S(g)+CO2(g) ��H2=-35kJ/mol��
������Ӧ�ų����������٣����������Ӧ������ѧ�����ƾ�����
(2)��COS(g)+H2(g)![]() H2S(g)+CO(g) ��H1=-17kJ/mol��
H2S(g)+CO(g) ��H1=-17kJ/mol��
��COS(g)+H2O(g)![]() H2S(g)+CO2(g) ��H2=-35kJ/mol��
H2S(g)+CO2(g) ��H2=-35kJ/mol��
���ݸ�˹���ɼ����![]() ��õ���CO(g)+H2O(g)
��õ���CO(g)+H2O(g)![]() H2(g)+CO2(g)����H=-18kJ/mol��
H2(g)+CO2(g)����H=-18kJ/mol��
(3)�ʻ���������ˮ����������ϵ��ʼͶ�ϱȲ��䣬����ʻ�����ˮ������Ӧ��ѡ���ԵĹؼ������ǣ�ѡ���Ч�Ĵ�����
(4)���ڳ��д����ĺ�ѹ�ܱ�������ֻ���з�ӦCOS(g)+H2(g)![]() H2S(g)+CO(g) ��H1=-17kJ/mol������ʼ�����n(H2)��n(COS)=m��mԽ����������Խ�࣬�����COS��ת���ʣ���m1>m2��
H2S(g)+CO(g) ��H1=-17kJ/mol������ʼ�����n(H2)��n(COS)=m��mԽ����������Խ�࣬�����COS��ת���ʣ���m1>m2��
���¶ȸ���T0��COSת���ʼ�С�Ŀ���ԭ��Ϊ��i�и�Ӧ������ii.����������ͣ�iii.ƽ��������У�
(5)�ڳ��д����ĺ�ѹ�ܱ������н��з�Ӧ��.COS(g)+H2O(g)![]() H2S(g)+CO2(g) ��H2
H2S(g)+CO2(g) ��H2
������ӦΪ���ȷ�Ӧ������ƽ��������У���ѧƽ�ⳣ����С����M���Ӧ��ƽ�ⳣ��<Q���ƽ�ⳣ����
��N��COSת����Ϊ60%��COS(g)��H2O(g)Ͷ�ϱ�1��1��
COS(g)+H2O(g)![]() H2S(g)+CO2(g)
H2S(g)+CO2(g)
��ʼ��(mol) 1 1 0 0
�仯��(mol)0.6 0.6 0.6 0.6
ƽ����(mol) 0.4 0.4 0.6 0.6![]()
��Ӧ��ƽ��������COS(g)���ʵ�������![]() =20%��
=20%��
��M��COSת����Ϊ60%��COS(g)��H2O(g)Ͷ�ϱ�1��3��N��COSת����Ϊ60%��COS(g)��H2O(g)Ͷ�ϱ�1��1����Ӧ��������ʵ�����ͬʱ����Ӧǰ���������ʵ������䣬��M���Q���Ӧ��ƽ��������������ʵ���֮��Ϊ1��1��

����Ŀ��һ���¶��£��������ݻ���Ϊ2.0 L�ĺ����ܱ������з�����Ӧ��2NO(g)��2CO(g)![]() N2(g)��2CO2(g)������������ʼ���ʵ����뷴Ӧ�¶����±���ʾ����Ӧ�����мס���������CO2�����ʵ�����ʱ��仯��ϵ��ͼ��ʾ��
N2(g)��2CO2(g)������������ʼ���ʵ����뷴Ӧ�¶����±���ʾ����Ӧ�����мס���������CO2�����ʵ�����ʱ��仯��ϵ��ͼ��ʾ��
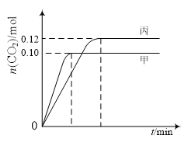
���� | �¶�/�� | ��ʼ���ʵ���/mol | |
NO (g) | CO (g) | ||
�� | T1 | 0.20 | 0.20 |
�� | T1 | 0.30 | 0.30 |
�� | T2 | 0.20 | 0.20 |
����˵����ȷ����
A. �÷�Ӧ������ӦΪ���ȷ�Ӧ
B. �ﵽƽ��ʱ������CO2����������ȼ��е�С
C. T1��ʱ������ʼʱ����г���0.40 mol NO��0.40mol CO��0.40mol N2��0.40mol CO2����Ӧ�ﵽ��ƽ��ǰv(��)��v(��)
D. T2��ʱ������ʼʱ����г���0.06molN2��0.12 molCO2�����ƽ��ʱN2��ת���ʴ���40%











