��Ŀ����
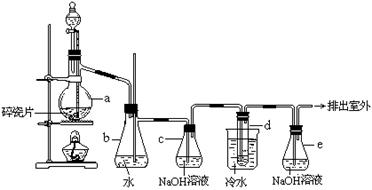
ij��ȤС���ͬѧ��ͬ��������ͼ��ʾ��ʵ��װ�ã��ȿ�������ȡ�����ֿ�������֤���ʵ����ʡ���ش��������⣺
��1������װ�â���ȡ���壬��������ķ�ӦӦ�߱��������� ��
��2������װ�â���ȡ���壨K2�رգ�K1������ͬѧ��Ϊ������װ�â�����ռ�H2��NH3�����壬�������ռ�O2��NO�����壬�������ǣ�
_______________________________________________________________________��
��ͬѧ��Ϊ����װ�â������Ľ������ı�����װ�ã���Ҳ���ռ�O2��NO�����壬�������ռ�NO2���壬�Ľ��ķ����� �������ռ�NO2�����ԭ���ǣ��û�ѧ����ʽ��ʾ�� ��
��3������װ�â� ��֤���ʵ����ʣ�K2��K1�رգ�����Ҫ���ʵ��֤��������
KMnO4��Cl2��Br2�������A �м�Ũ���ᣬB�м� ��C�м� ���۲쵽C�е������� ��
����1����2������ÿ��2�֣���3��ÿ��1�֡���1����+Һ��Ӧ���������
��2��O2���ܶȱȿ����� NO�ܶ�������������NO����O2��Ӧ ��
��װ�â��м���ˮ �� NO2����H2O������Ӧ ��3��KMnO4���� ��NaBr��Һ ����Һ���ɫ ��
���������������1������װ�õ��ص��֪��װ�â��������Ʊ���+Һ��Ӧ���Ҳ�����ȵ�������Ʊ���
��2����ͬѧ��O2���ܶȴ��ڿ������ܶȣ���������Ӧ���������ſ������ռ���һ�������������ܷ�����Ӧ���ɶ���������һ������Ӧ������ˮ���ռ������Ը�װ�ò����ռ�������һ��������
��ͬѧ������������ˮ�ܷ�����Ӧ�����ܲ�����ˮ���ռ��������������ܶȴ��ڿ������ܶȣ�����Ӧ�������ѻӷ����л��ܼ��������ſ������ռ�����װ�ò��ı�����װ�ã�����ֻ�ܲ������ѻӷ����л��ܼ����ռ���
��3������������+��ԭ������������+��ԭ��������ԣ������������������ԭ�ԣ���ԭ������ԭ������Ҫ֤�������ԣ�KMnO4��Cl2��Br2���ɽ�KMnO4��Ϊ�����������ᷴӦ�ж��Ƿ����������������������Դ���Br2��������ͨ��NaBr��Һ�У������Һ��Ϊ�Ⱥ�ɫ��˵����Br2���ɣ��Ӷ�ȷ�������ԣ�KMnO4��Cl2��Br2��
���㣺���鳣��������Ʊ����ռ��Լ�������ԭ��Ӧ���й�Ӧ��
�����������Ǹ߿��еij������ͣ����ڻ���������Ŀ��顣���������߿������ض�ѧ��ʵ������������������Ҫע�������������ʼ��ܶ�ѡ����Ӧ���ռ������Լ�ʵ����Ƶķ�������ơ�����������Ҫ���Գ���������ѡ�á�ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ�Լ��������֪ʶ���ʵ�������������

ijѧ����ʵ������ȡCl2ʱ���ܽ������²����������Ӻ�װ�ü�������ԣ��ڻ������ȣ��ۼ���MnO2��ĩ�����ɷ�Һ©������ƿ���Ũ����ݶ����Cl2��NaOH���գ����������ſ������ռ�Cl2������ȷ����˳����
| A���٢ڢۢܢݢ� | B���ۢܢڢ٢ޢ� |
| C���٢ۢܢڢޢ� | D���ۢ٢ܢڢޢ� |
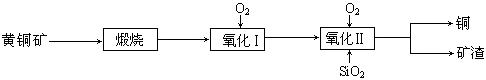
���̿��к�MnO2Լ70%��SiO2Լ20%��Al2O3Լ4%������Ϊˮ�֣���п���к�ZnSԼ80%��FeS��CuS��SiO2��Լ7%������Ϊˮ�֡�������Ա�������ۺ�������������Դ��ͬ��������գ���ȡZn��MnO2��Na2SO4���乤���������£�
��1��I����Һ�к���MnSO4��ZnSO4��CuSO4��Fe2(SO4)3��Al2(SO4)3�ȡ�д��MnO2��CuS�����ᷴӦ�Ļ�ѧ����ʽ�� ��
��2����֪Fe(OH)3��Al(OH)3��Zn(OH)2�������ʿ�ʼ��������ȫ����ʱ��Һ��pH���±���
| ������ | Fe(OH)3 | Al(OH)3 | Zn(OH)2 |
| ��ʼ����pH | 2.3 | 4.0 | 5.6 |
| ��ȫ����pH | 4.1 | 5.2 | 8.0 |
��III�е�����Һ��pH��5.2��5.4����ʱ���ɳ���M�ijɷ�Ϊ ��д��ѧʽ����III�м���MnO2�������� ��
��3��Na2SO4��Na2SO4��10H2O���ܽ�����ߣ�g/100gˮ����ͼ����IV�еõ�Na2SO4����IJ����ǣ��������MnCO3��ZnCO3�����Һ���½ᾧ�� �����Ҵ�ϴ�Ӻ������Ҵ�ϴ�Ӷ�����ˮϴ��ԭ���� ��

��4��V���ö��Ե缫����Ƶ�Zn��MnO2���������ĵ缫��ӦʽΪ ��
��5����ɫ��ѧ˼���ڱ������еõ��˳�����֣��ڱ����������п�ѭ��ʹ�õ���Ҫ�����У�MnO2��ZnCO3��MnCO3�� �� ��д��ѧʽ����
��15�֣�ij�о�С���û�ͭ����Ҫ�ɷ���CuFeS2������SΪ-2�ۣ�Ϊ��Ҫԭ����ͭ�����ܷ�ӦΪ��2CuFeS2+2SiO2+5O2��2Cu+2FeSiO3+4SO2����ʵ�ϸ÷�Ӧ�ǰ��������̷ֲ����еģ�

��1��������ķ�Ӧ��Ҫ���������ɵ�����������һ������Ϊ��������������������跴Ӧ���ɿ�������������Ҫ�ɷ��� ���ѧʽ����
��2���ݱ�������һ��ϸ�������������¿��Խ���ͭ�������������Σ���Ӧ����������Һ�з����ġ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3���ҹ�ѧ���о����֣��Ծ�CuFeS2��Ϊԭ���ڷ���¯����O2����������Ӧ����������ȴ���ܽ⡢�������ᾧ���õ�CuSO4��5H2O�������ɱ��ܹ��������ࡣ�й�ʵ�������±���
| ����¯�¶�/�� | 560 | 580 | 600 | 620 | 640 | 660 |
| ˮ����Cu/% | 90.12 | 91.24 | 93.50 | 92.38 | 89.96 | 84.23 |
| ������Cu/% | 92.00 | 93.60 | 97.08 | 97.82 | 98.16 | 98.19 |
��ʵ�����������з���¯�¶�Ϊ600��620 �档������¶ȵķ����� ��
�۵��¶ȸ���600��620 ��ʱ����������ˮ����ͭ�½���ԭ���� ��
����������ȴ��ij�����ʵ�����������Ҫ�� ����֪����Һ�У�Cu2+��ʼ�����ͳ�����ȫ��pH�ֱ�Ϊ4.7��6.7��Fe3+��ʼ�����ͳ�����ȫ��pH�ֱ�Ϊ1.1��3.2������Ƶõ�����ͭ��Һ�к���������Fe3+����д����ȥ��Һ��Fe3+��ʵ��������裺 ��
[14��]ʵ�����Ʊ�����ͪ�Ļ�ѧ����ʽΪ��
�Ʊ������л���
 �ȸ���Ӧ��
�ȸ���Ӧ��
��Ҫʵ��װ�úͲ������£�
��I���ϳɣ�������ƿ�м���20 g��ˮAlCl3��30 mL��ˮ����Ϊ���ⷴӦҺ���¹��죬�߽���������μ�6 mL��������10 mL��ˮ���Ļ��Һ�����Ƶμ����ʣ�ʹ��ӦҺ�����������μ���Ϻ���Ȼ���1Сʱ��
���������ᴿ��
�ٱ߽���������μ�һ����Ũ�������ˮ���Һ������õ��л���
��ˮ���ñ���ȡ����Һ
�۽��٢������л���ϲ���ϴ�ӡ������ȥ�����õ�����ͪ�ֲ�Ʒ
������ֲ�Ʒ�õ�����ͪ���ش��������⣺
��1������a�����ƣ�____________��װ��b�����ã�________________________________��
��2���ϳɹ�����Ҫ����ˮ������������____________________________________________��
��3�������������ͱ��Ļ��Һһ���Ե�������ƿ�����ܵ���_________________��
| A����Ӧ̫���� | B��Һ��̫������� | C����Ӧ�仺�� | D������������ |
��5����Һ©��ʹ��ǰ��___________________��ϴ�����á���ȡʱ���Ⱥ�������ȡҺ����ȡ��������ҡ��________________����Һ©����������̨�������Ͼ���Ƭ�̣��ֲ㡣�������²�Һ��ʱ��Ӧ��________________��Ȼ������ų��²�Һ�壬�ϲ�Һ����Ͽڵ�������6���ֲ�Ʒ�����ᴿʱ������װ�����¶ȼ�λ����ȷ����________________�����ܻᵼ���ռ����IJ�Ʒ�л��еͷе����ʵ�װ����________________��



 6LiBr��2H2O��N2����4CO2������Ҫ�����������£�
6LiBr��2H2O��N2����4CO2������Ҫ�����������£�