题目内容
7.有一瓶无色溶液,可能含有Na+、K+、Al3+、Mg2+、NH4+、Cl-、SO42-、HCO3-、Ba2+、MnO4-中的几种.为确定其成分,进行如下实验:①取少许溶液,逐渐加入过量的Na2O2固体,产生无色无味的气体和白色沉淀,且白色沉淀逐渐增多后又部分溶解;
②另取部分溶液,加入HNO3酸化的Ba(NO3)2溶液,有白色沉淀产生;
③用洁净的铂丝蘸取原溶液在酒精灯火焰上灼烧,观察到黄色火焰,
下列推断正确的( )
| A. | 由①只能确定溶液中有Al3+、Mg2+,没有HCO3- | |
| B. | 由②知溶液中有SO42-,没有Ba2+ | |
| C. | 由③确定溶液中有Na+,没有K+ | |
| D. | 若检验溶液中是否有存在Cl-,可取少许原溶液,直接向其中加入AgNO3溶液 |
分析 过氧化钠和水反应生成氢氧化钠和氧气,根据氢氧化镁沉淀不溶于氢氧化钠溶液中,氢氧化铝能溶于氢氧化钠中,硫酸钡为白色不溶于硝酸的白色沉淀.
①取部分溶液,加入过量Na2O2固体,产生无色无味的气体和白色沉淀,白色沉淀逐渐增多后又部分溶解,则沉淀的成分是氢氧化镁和氢氧化铝;
②取部分溶液,加入HNO3酸化的Ba(NO3)2溶液,有白色沉淀产生,白色不溶于硝酸的白色沉淀是硫酸钡沉淀;
③焰色反应为黄色说明含有钠元素;
据此来回答问题.
解答 解:该溶液是无色溶液,则一定不会含有高锰酸跟离子.
①取部分溶液,加入过量Na2O2固体,过氧化钠先是和水反应生成氢氧化钠和氧气,产生无色无味的气体是氧气,一定不是氨气,说明不含铵根离子;白色沉淀逐渐增多后又部分溶解,则沉淀的成分是氢氧化镁和氢氧化铝,则证明其中一定含有镁离子和铝离子,一定不含有铵根离子、碳酸氢根离子(和铝离子不共存);
②取部分溶液,加入HNO3酸化的Ba(NO3)2溶液,有白色沉淀产生,和硫酸根离子反应生成白色不溶于硝酸的白色沉淀是硫酸钡沉淀,证明一定含有硫酸根离子,一定无钡离子;
③用洁净的铂丝蘸取原溶液在酒精灯火焰上灼烧,观察到黄色火焰,说明是钠元素的性质,原溶液中含有钠离子;
综上所述:原溶液中一定不含:NH4+、HCO3-、MnO4-、Ba2+;一定含有:Na+、Al3+、Mg2+、SO42-;不能确定的是K+、Cl-;
A、由①能确定溶液中有Al3+、Mg2+,没有HCO3-和NH4+,故A错误;
B、由②知溶液中有SO42-,由于有SO42-则没有Ba2+,故B正确;
C、K+的存在不能确定,故C错误;
D、由于溶液中有SO42-,能干扰氯离子的检验,故D错误.
故选B.
点评 本题考查学生常见离子的检验方法,可以根据所学的知识来回答,难度不大.

 名校课堂系列答案
名校课堂系列答案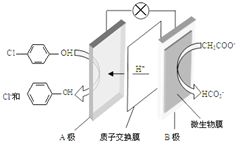 现在污水治理越来越引起人们重视,可以通过膜电池除去废水中的乙酸钠和对氯苯酚(
现在污水治理越来越引起人们重视,可以通过膜电池除去废水中的乙酸钠和对氯苯酚(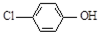 ),其原理如图所示,下列说法正确的是( )
),其原理如图所示,下列说法正确的是( )| A. | 当外电路中有0.2mole-转移时,通过质子交换膜的H+的个数为0.2NA | |
| B. | A极的电极反应式为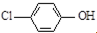 +e-═Cl-+ +e-═Cl-+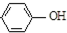 | |
| C. | 电流方向从B极沿导线经小灯泡流向A极 | |
| D. | B为电池的正极,发生还原反应 |
| A. | 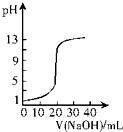 如图表示0.10 mol•L-1 NaOH溶液滴定20.00 mL 0.10 mol•L-1醋酸溶液的滴定曲线 | |
| B. | 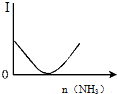 如图表示乙酸溶液中通入氨气至过量过程中溶液导电性I的变化 | |
| C. | 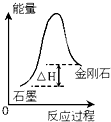 根据如图所示可知:石墨比金刚石稳定 | |
| D. | 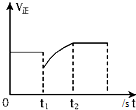 如图表示反应2SO2+O2?2SO3,t1时刻只减小了SO3的浓度 |
| A. | 由于Ksp(ZnS)>Ksp(CuS),所以ZnS在一定条件下可转化为CuS | |
| B. | 已知AgCl的Ksp=1.8×10-10,将0.01 mol•L-1KCl溶液和等体积的0.01 mol•L-1 AgNO3溶液混合,有AgCl沉淀析出 | |
| C. | 常温下,为确定某酸H2A是强酸还是弱酸,可测NaHA溶液的pH,若pH>7,则H2A是弱酸;若pH<7,则H2A是强酸 | |
| D. | pH=8的Ba(OH)2溶液和pH=8的氨水中,由水电离的c(OH-)均为1×10-8 mol•L-1 |
| A. | Na2CO3 是碱 | B. | Na2C03 是盐 | C. | Na2C03是钠盐 | D. | Na2CO3是碳酸盐 |