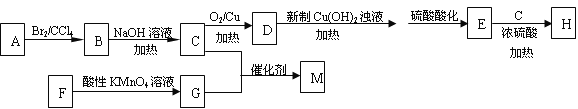
��Ŀ����
����Ŀ������ѧ��-ѡ��2����ѧ�뼼����
�ʰ������������[��ѧʽΪ(NH2CH2COO)2Fe]�dz��õIJ���������ϳɷ������£�
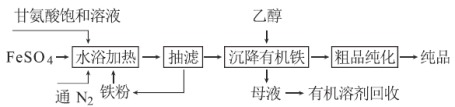
��1��ͨ��N2�������� ��
��2����֪�ʰ����������ԣ���ṹ��ʽΪNH2CH2COOH���ʰ������������������ˮ����ˮ���ѵ��룬д����ˮԡ���ȡ������������ʰ����������������Ӧ�����ӷ���ʽ ��
��3�����˵ĺô��� ���ӡ�ĸҺ���л����л��ܼ��ķ����� ��
��4������Ʒ�������IJ���Ϊ����ˮϴ�ӡ� ϴ�ӡ�������и�������ʹ�����豸���ѡ�� �����ѹ�����������ո����������
��5��������ָ�������ڡ�ˮԡ���ȡ�������Ͷ��������ʯ��ʯ������ͬʱ��߲�Ʒ�IJ��ʺʹ��ȣ������ԭ�� ��
��6�����ʰ����Ͷ����Ϊ300 kg��������Ʒ346.8 kg�������Ϊ �����ʰ������Է�������Ϊ75��
��������1����ֹFe2+����������������Լӿ컯ѧ��Ӧ���ʡ�
��2��2NH2CH2COOH��Fe2��![]() (NH2CH2COO)2Fe��2H����
(NH2CH2COO)2Fe��2H����
��3���ӿ���˵��ٶȣ�����4����ˮ�Ҵ������ͪ�ȣ�����ո������
��5��ʯ��ʯ����H����ʹH��Ũ�Ƚ��ͣ������ڷ�Ӧ�����ɸʰ������������ķ�����У�ͬʱ��Ca2����SO42����Ӧ����CaSO4��������������������SO42����Ũ�ȣ�����6��85����
��������
�����������1���ʰ��ᱥ����Һͨ�뵪��������ͨ����Һ�����������Һ�����ã������Լӿ컯ѧ��Ӧ���ʣ�ˮԡ���ȸʰ��ᱥ����Һ�����������Ļ������������ױ���������������Ϊ��������ͨ�뵪������ֹFe2+������������
��2���ʰ����������ԣ��������۷�����Ӧ2NH2CH2COOH��Fe��(NH2CH2COO)2Fe��H2�������ˮԡ���ȸʰ��ᱥ����Һ�����������Ļ��������ӷ�Ӧ����ʽΪ2NH2CH2COOH��Fe2��![]() (NH2CH2COO)2Fe��2H����
(NH2CH2COO)2Fe��2H����
��3��������ָ�ó�����ʹƿ�е�ѹǿ���ͣ��ﵽ��Һ�����Ŀ�ģ��ܼӿ���˵��ٶȣ����ܵ�Һ���������ķ������룬���Բ�������ķ�����ĸҺ�л����л��
��4���ʰ������������Ϊ�л�������Ʒ����������ˮ�Ҵ������ͪ�ȣ�ϴ�ӣ��л���ķе�ϵͣ�������ո������ʵ���ڵ��������¼�����ˮ�������ѡ����ո�������
��5���ʰ����������ԣ���ṹ��ʽΪNH2CH2COOH��Ͷ��������ʯ��ʯ��ʯ��ʯ����H+��ʹH+Ũ�Ƚ��ͣ������ڷ�Ӧ2NH2CH2COOH+Fe2+![]() ��NH2CH2COO��2Fe+2H+�������ɸʰ������������ķ�����У�ͬʱ��Ca2+��SO42-��Ӧ����CaSO4��������������������SO42-��Ũ�ȣ�
��NH2CH2COO��2Fe+2H+�������ɸʰ������������ķ�����У�ͬʱ��Ca2+��SO42-��Ӧ����CaSO4��������������������SO42-��Ũ�ȣ�
��6���ʰ����Ͷ����Ϊ300kg���ʰ�������ʵ���n��300000g��75g/mol��4000mol������n[��NH2CH2COO��2Fe]=2000mol�������ʣ�![]() ��100%��=85%��
��100%��=85%��

 ����������ϵ�д�
����������ϵ�д� �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�