��Ŀ����
����Ŀ����ҩ��C��������N�Լ��߷�����֬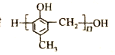 �ĺϳ�·�����£�
�ĺϳ�·�����£�

��1��A�Ļ�ѧ����Ϊ_____��A�ڴ��������¿���H2��Ӧ����B��B��ʽ��Ϊ108��B�Ľṹ��ʽΪ_____��
��2��C�й����ŵ�����Ϊ_____��C�ڼ���������ˮ��Ļ�ѧ��Ӧ����ʽΪ_____��
��3��A��������Һ��Ӧ�Ļ�ѧ����ʽΪ_________��
��4��F���ȩ�ϳɸ߷�����֬�ķ�Ӧ����Ϊ_________��N�Ľṹ��ʽΪ________��
��5���������һ��ͬ���칹����������������
����FeCl3��Һ��������ɫ��Ӧ
���ܷ���������Ӧ��������������ˮ�����֮һ����FeCl3��Һ������ɫ��Ӧ
�۷�������5�ֲ�ͬ��ѧ�������⣬�˴Ź����������֮��Ϊ��1:2:2:1.
д����ͬ���칹��Ľṹ��ʽ________��
��6��д�����Ҵ�Ϊԭ���Ʊ�������CH3COCl�ĺϳ�·��_________��
�ϳ�·������ͼʾ�����£�
![]()
���𰸡� ����ȩ ![]() ���� �ǻ�
���� �ǻ� ![]()
![]() ���۷�Ӧ
���۷�Ӧ 
 CH3CH2OH
CH3CH2OH ![]() CH3CHO
CH3CHO ![]() CH3COOH
CH3COOH![]() CH3COCl
CH3COCl
�����������⿼���л���ĺϳɺ��ƶϣ���1��������Ŀ��������Ϣ��A�к��м�ȩ��������ϢI�ͱ�����Ľṹ��ʽ���Ƴ�A�Ľṹ��ʽΪ![]() ������Ϊ����ȩA�ڴ����������������������ӳɷ�Ӧ���������ɱ��״���Ҳ��������
������Ϊ����ȩA�ڴ����������������������ӳɷ�Ӧ���������ɱ��״���Ҳ��������![]() ������B��ʽ������B�Ľṹ��ʽΪ
������B��ʽ������B�Ľṹ��ʽΪ![]() ����2��������ͱ��״�����������Ӧ�����C�к��еĹ��������������ǻ���C�ڼ��������·���ˮ�ⷴӦ�����ɴ����ᣬ����Ӧ����ʽΪ
����2��������ͱ��״�����������Ӧ�����C�к��еĹ��������������ǻ���C�ڼ��������·���ˮ�ⷴӦ�����ɴ����ᣬ����Ӧ����ʽΪ![]() ����4�����ݸ߷��ӻ�����Ľṹ��ʽ���䵥���Ǽ�ȩ�ͶԼ����ӣ�����γɸ߷��ӻ����������Ϊ���۷�Ӧ��F�Ľṹ��ʽΪ
����4�����ݸ߷��ӻ�����Ľṹ��ʽ���䵥���Ǽ�ȩ�ͶԼ����ӣ�����γɸ߷��ӻ����������Ϊ���۷�Ӧ��F�Ľṹ��ʽΪ ��A��������Һ��Ӧ�Լ������������£�����D��DΪ�����ᣬ������ϢII���Ƴ�E�Ľṹ��ʽΪ
��A��������Һ��Ӧ�Լ������������£�����D��DΪ�����ᣬ������ϢII���Ƴ�E�Ľṹ��ʽΪ![]() �����N�Ľṹ��ʽΪ
�����N�Ľṹ��ʽΪ ����5�����ݢ�˵���������ǻ������ݢ����ڼ���ij�����ṹ��ʽ�д��ڡ�
����5�����ݢ�˵���������ǻ������ݢ����ڼ���ij�����ṹ��ʽ�д��ڡ� ��������5�ֲ�ͬ��������ԭ�ӣ���ԭ�Ӹ�����Ϊ1��2��2��1����ṹ��ʽΪ
��������5�ֲ�ͬ��������ԭ�ӣ���ԭ�Ӹ�����Ϊ1��2��2��1����ṹ��ʽΪ ����6��������Ϣ���Ʊ�CH3COCl��Ӧ����CH3COOH��SOCl2��Ӧ�������Ҫ���Ҵ��Ʊ����ᣬ��˺ϳ�·����CH3CH2OH
����6��������Ϣ���Ʊ�CH3COCl��Ӧ����CH3COOH��SOCl2��Ӧ�������Ҫ���Ҵ��Ʊ����ᣬ��˺ϳ�·����CH3CH2OH ![]() CH3CHO
CH3CHO ![]() CH3COOH
CH3COOH![]() CH3COCl��
CH3COCl��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�

