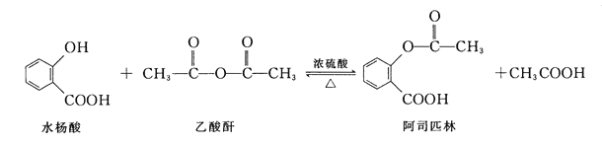
��Ŀ����
����Ŀ��������Ҫ��ս�����ʣ�2016���ҹ�����ʡ���ֳ������ٿ��ٴ�ˢ���ٿ����������¼������������Ҫ�ɷ���![]() ������������
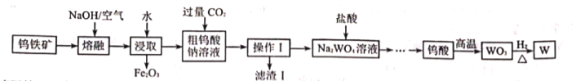
������������![]() ����ȡ�ٵĹ�ҵ�������£���֪�������£�����������ˮ����
����ȡ�ٵĹ�ҵ�������£���֪�������£�����������ˮ����
��ش������й����⣺
��1����ȡ����еķ��������������________��
��2������![]() ����Ҫ�ɷ���________���ѧʽ����д�����ɸ����ʵ����ӷ���ʽ________��
����Ҫ�ɷ���________���ѧʽ����д�����ɸ����ʵ����ӷ���ʽ________��
��3����֪![]() ����Ԫ�صĻ��ϼ�Ϊ+2�ۣ�
����Ԫ�صĻ��ϼ�Ϊ+2�ۣ�![]() �����ڹ����з�����Ӧ�Ļ�ѧ����ʽΪ________��
�����ڹ����з�����Ӧ�Ļ�ѧ����ʽΪ________��
��4����![]() ��Һ�м�������ʱ��Ϊ�˷�ֹ�ֲ���ȹ���Ӧ��ȡ�Ĵ�ʩ��________��
��Һ�м�������ʱ��Ϊ�˷�ֹ�ֲ���ȹ���Ӧ��ȡ�Ĵ�ʩ��________��
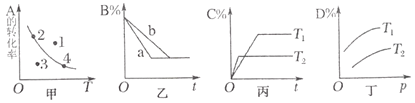
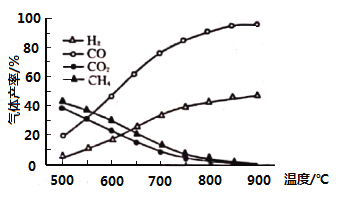
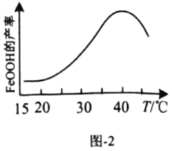
��5����ͼΪ![]() �ڳ����µij����ܽ�ƽ�����ߣ�����
�ڳ����µij����ܽ�ƽ�����ߣ�����![]() ��
��![]() ��Һ��
��Һ��![]() ��
��![]() ��Һ�������2:1��ϣ�Ҫ����
��Һ�������2:1��ϣ�Ҫ����![]() ��������
��������![]() ________����������Һ����仯��
________����������Һ����仯��
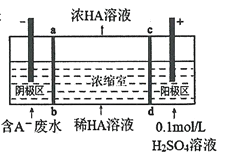
��6����̼����![]() ���������������������
���������������������![]() ��Һ�����Һ��ͨ�������Ի����٣������������������Ტ�ų�
��Һ�����Һ��ͨ�������Ի����٣������������������Ტ�ų�![]() ����������ӦʽΪ________�����ڱ�״���·ų�
����������ӦʽΪ________�����ڱ�״���·ų�![]() �����������ص�����Ϊ________
�����������ص�����Ϊ________![]() ��
��
���𰸡����� ![]()
![]()
 �ִλ����������ᣬ �����Ͻ���
�ִλ����������ᣬ �����Ͻ��� ![]()
![]() 40.5
40.5
��������
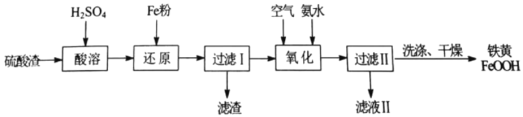
�����̿�֪�������������������ơ�������Ӧ�����������������ƣ�Al2O3���������Ʒ�Ӧ����NaAlO2��ˮ��ˮ��ʱ��������������ˮ������������ˮ���ʹ��˺�õ�����Һ�������ƺ�NaAlO2����������Ҫ�ɷ���Fe2O3����Һ��ͨ��CO2����NaAlO2��Ӧ����Al(OH)3������̼�����ƣ������������õ���������Һ�����������ʲ������ǹ��ˣ���Һ�ٺ�Ũ���ᷴӦ����������Ȼ��ƣ���������ֽ�����������ٺ�ˮ���û�ԭ����ԭ�������������٣��ݴ��ٽ��н��⡣
��1���ɷ�����֪����ȡ����еķ���õ�Fe2O3����Һ���ʸ÷�������������ǹ��ˣ��ʴ�Ϊ�����ˣ�
��2������������ͨ��CO2��Ҫ������ӦΪ��NaAlO2+2H2O+CO2=Al(OH)3��+NaHCO3��������![]() ����Ҫ�ɷ���Al(OH)3�����ɸ����ʵ����ӷ���ʽ
����Ҫ�ɷ���Al(OH)3�����ɸ����ʵ����ӷ���ʽ![]() ���ʴ�Ϊ��Al(OH)3��
���ʴ�Ϊ��Al(OH)3��![]() ��
��
��3����������ͼ��֪��FeWO4�����ڹ����е÷�Ӧ����FeWO4��NaOH��O2����������Na2WO4��Fe2O3�ȣ�����FeWO4����Ԫ�صĻ��ϼ�Ϊ+2�ۣ��ʸ÷�ӦΪ������ԭ��Ӧ���ʷ�����Ӧ�Ļ�ѧ����ʽΪ��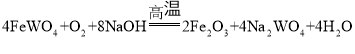 ���ʴ�Ϊ��
���ʴ�Ϊ��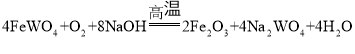 ��
��
��4����Na2WO4��Һ�м�������ʱ��Ϊ�˷�ֹ�ֲ���ȹ�������Na2WO4�������ܹ���ʱ��ȫ��Ӧ����Ӧ��ȡ�Ĵ�ʩ�Ƿִλ����������ᣬ �����Ͻ��裬�ʴ�Ϊ���ִλ����������ᣬ �����Ͻ��裻
��5����CaWO4�ڳ����µij����ܽ�ƽ�����߿�֪��![]() ������
������![]() ��Na2WO4��Һ��1��10-4mol/L��CaCl2��Һ�������2:1��Ϻ���Һ��c(Ca2+)=
��Na2WO4��Һ��1��10-4mol/L��CaCl2��Һ�������2:1��Ϻ���Һ��c(Ca2+)=![]() ��Ҫ����CaWO4������c(Ca2+)c(
��Ҫ����CaWO4������c(Ca2+)c(![]() )��
)��![]() ����
����![]() �����
�����![]()
![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��6��![]() �������� ��������������Ӧ�������������������Ტ�ų�
�������� ��������������Ӧ�������������������Ტ�ų�![]() �� ��������ӦʽΪ��
�� ��������ӦʽΪ��![]() ����״��������
����״��������![]() �����뷴Ӧ��
�����뷴Ӧ�� ![]() Ϊ
Ϊ![]() �� ������
�� ������ ![]() ת��Ϊ
ת��Ϊ![]() �� ���ص�����Ϊ��
�� ���ص�����Ϊ�� ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��40.5��
��40.5��

����Ŀ��ijʵ���ҷ�Һ��![]() �����ӣ���ͨ���������̱��Ϊ���Ʊ�
�����ӣ���ͨ���������̱��Ϊ���Ʊ�![]() ��
��
��֪��(a)![]()
![]() (��ɫ)
(��ɫ)
(b)����������������������pH�������
�������� | pH | |
���� | ���� | |
Fe3+ | 2.7 | 3.7 |
Cr3+ | 4.9 | 6.8 |
��ش�
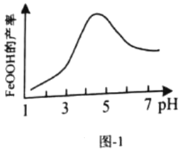
(1)�����Թ��м�������(NH4)2Cr2O7���壬�μ�����ŨKOH��Һ�����ȣ��۲쵽����Ҫ�����ǣ�_________________��
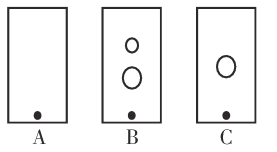
(2)ijͬѧ����ֽ�������жϲ���ټ���KOH�����Ƿ���ʡ��ڼ���һ����KOH��Һ��,��ëϸ��ȡ��������������ɫ��չ������Ѭ��ĵ���ͼ��ʾ������KOH���ʺϵ�ʵ������(��ͼABC��ѡ��ʵ��˳������)_________________,C�İߵ���ɫΪ_________________��
(3)����ں�Cr���ʷ�������Ҫ��Ӧ�����ӷ���ʽΪ_________________��
(4)������װ���У������Ӧѡ��ʵ��װ����_________________��(����)
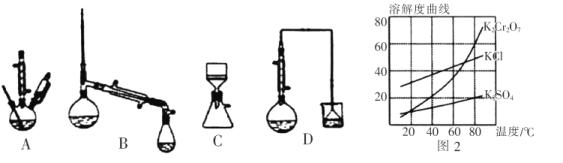
(5)�������ʵ��ܽ��������ͼ2,����ݿ����õ����в��ֲ���:a.���������ִ������壬ֹͣ����;b.��ȴ������;c.��������Һ���־�Ĥ��ֹͣ����;d.ϴ��;e.���ȹ���;f.���ˡ���ѡ����ʲ�������ȷ˳��_________________��
(6)������к��ʵ�ϴ�Ӽ���_________________(����ˮ�Ҵ��������Ҵ�-ˮ���Һ��������ˮ��������ˮ��),�ֲ�Ʒ��һ���ᴿ�ķ�����_________________��
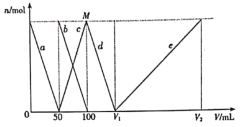
(7)ȡmg�ֲ�Ʒ���250mL��Һ��ȡ25.00mL����ƿ�У���cmol.L-1��![]() ����Һ�ζ�(���ʲ���Ӧ)�����ı�
����Һ�ζ�(���ʲ���Ӧ)�����ı�![]() ��ҺVmL����ôֲ�Ʒ��
��ҺVmL����ôֲ�Ʒ��![]() �Ĵ���Ϊ_________________��(
�Ĵ���Ϊ_________________��(![]() ��ʽ��:294)
��ʽ��:294)