��Ŀ����
7����һ����Ҫ��������1�����������У�δ�ɶԵ���������ԭ�ӣ�����Χ�����Ų�ʽΪ��3d54s1��
��2��3d�ܼ�Ϊ����������������ӣ������ӵĵ����Ų�ʽΪ1s22s22p63s23p63d5��
������A��B��C��D��E����Ԫ��Ϊ1��18��Ԫ�أ���֪�����ǵ�ԭ��������������A��B����Ԫ�صĺ˵����֮��������ǵ�ԭ������������֮�ͣ�Bԭ���������������������������2��CԪ��ԭ�ӵĵ��Ӳ�������������������EԪ�ص���1��D��E��ԭ������֮��Ϊ30�����������γɵĻ������мס��ҡ����������֣������ֻ�������ԭ�Ӹ��������±���
| �� | �� | �� | �� | |
| �������и�Ԫ��ԭ�Ӹ����� | A��C 1��1 | B��A 1��2 | D��E 1��3 | B��E 1��4 |
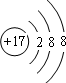 ��Bԭ�Ӻ�����2��δ�ɶԵ��ӣ����ǵ�������ȣ����ȡ�����ȡ�����
��Bԭ�Ӻ�����2��δ�ɶԵ��ӣ����ǵ�������ȣ����ȡ�����ȡ�������2�����ˮ��Һ�м���MnO2������ʵ�����Ʊ�C�ĵ��ʣ��仯ѧ����ʽ��2H2O2$\frac{\underline{\;MnO_{2}\;}}{\;}$2H2O+O2����
��3����֪�л����ҵķ���Ϊƽ��ṹ�����Ƕ�Ϊ120�㣬���Ľṹ��ʽΪCH2=CH2��
��4������ˮ��Һ�����ԣ��뱥��NaHCO3��Һ��Ӧ����������������������й����ӷ���ʽ��Al3++3HCO3-=Al��OH��3��+3CO2����
���� ��һ����1�����������У�δ�ɶԵ���������ԭ�ӣ�sd�ܼ�����5�����ӡ�4s�ܼ�����1�����ӣ�
��2��3d�ܼ�Ϊ����������������ӣ����ԭ�Ӽ۵����Ų�ʽΪ3d64s2������������ԭ����д�����ӵĵ����Ų�ʽ��
������A��B��C��D��E����Ԫ��Ϊ1��18��Ԫ�أ����ǵ�ԭ��������������Bԭ���������������������������2��ԭ��ֻ����2�����Ӳ㣬����������Ϊ4����BΪ̼Ԫ�أ�A��B����ԭ�ӵĺ˵����֮��������ǵ�ԭ������������֮�ͣ����߲�����ͬ���ڣ�A��ԭ������С��̼��ֻ�ܴ��ڵ�һ���ڣ�����֪AΪHԪ�أ�D��E��ԭ������֮��Ϊ30������ߴ��ڵ������ڣ�ΪAl��Cl��Si��S����B��E�γɵĻ����ﶡ��B��E=1��4����DΪAl��EΪCl��CԪ��ԭ�ӵĵ��Ӳ�������������������EԪ�ص���1����CΪOԪ�أ�����֪��ΪH2O2����ΪC2H4����ΪAlCl3����ΪCCl4���ݴ˽��
��� �⣺��һ����1�����������У�δ�ɶԵ���������ԭ�ӣ�sd�ܼ�����5�����ӡ�4s�ܼ�����1�����ӣ�����Χ�����Ų�ʽΪ��3d54s1��
�ʴ�Ϊ��3d54s1��
��2��3d�ܼ�Ϊ����������������ӣ����ԭ�Ӽ۵����Ų�ʽΪ3d64s2�����������ԭ����֪�����ӵĵ����Ų�ʽΪ��1s22s22p63s23p63d5��
�ʴ�Ϊ��1s22s22p63s23p63d5��
������A��B��C��D��E����Ԫ��Ϊ1��18��Ԫ�أ����ǵ�ԭ��������������Bԭ���������������������������2��ԭ��ֻ����2�����Ӳ㣬����������Ϊ4����BΪ̼Ԫ�أ�A��B����ԭ�ӵĺ˵����֮��������ǵ�ԭ������������֮�ͣ����߲�����ͬ���ڣ�A��ԭ������С��̼��ֻ�ܴ��ڵ�һ���ڣ�����֪AΪHԪ�أ�D��E��ԭ������֮��Ϊ30������ߴ��ڵ������ڣ�ΪAl��Cl��Si��S����B��E�γɵĻ����ﶡ��B��E=1��4����DΪAl��EΪCl��CԪ��ԭ�ӵĵ��Ӳ�������������������EԪ�ص���1����CΪOԪ�أ�����֪��ΪH2O2����ΪC2H4����ΪAlCl3����ΪCCl4��
��1��EΪ��Ԫ�أ�Cl-�����ӵĽṹʾ��ͼΪ ��BΪ̼Ԫ�أ�ԭ�Ӻ�������Ų�ʽΪ1s22s22p2��2p�ܼ��ϵ�2��������δ�ɶԵ��ӣ����ǵ�������ȣ�
��BΪ̼Ԫ�أ�ԭ�Ӻ�������Ų�ʽΪ1s22s22p2��2p�ܼ��ϵ�2��������δ�ɶԵ��ӣ����ǵ�������ȣ�
�ʴ�Ϊ�� ��2����ȣ�
��2����ȣ�
��2����ף�H2O2����ˮ��Һ�м���MnO2������ʵ�����Ʊ��������仯ѧ����ʽ��2H2O2$\frac{\underline{\;MnO_{2}\;}}{\;}$2H2O+O2�����ʴ�Ϊ��2H2O2$\frac{\underline{\;MnO_{2}\;}}{\;}$2H2O+O2����
��3���л����ң�C2H4���ķ���Ϊƽ��ṹ�����Ƕ�Ϊ120�㣬���Ľṹ��ʽΪCH2=CH2���ʴ�Ϊ��CH2=CH2��
��4������AlCl3����ˮ��Һ�����ԣ��뱥��NaHCO3��Һ��Ӧ�����������������������������̼��������ӷ���˫ˮ����������������������̼�����ӷ���ʽ��Al3++3HCO3-=Al��OH��3��+3CO2����
�ʴ�Ϊ��Al3++3HCO3-=Al��OH��3��+3CO2����
���� ���⿼��ṹ����λ�ù�ϵӦ�ã�Ԫ���ƶ��ǽ���Ĺؼ���ע�������ö���������Ԫ�ؽ����ƶϣ��Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | 1��1��1 | B�� | 3��2��1 | C�� | 6��3��2 | D�� | 1��2��3 |
| ��ʵ | ���� | |
| A | Aԭ�ӡ�Bԭ�Ӽ۵������ֱ�Ϊ1��2 | A�Ľ�����һ����B��ǿ |
| B | ij��̬����X��H2ͨ�������²��ܹ��� | �ڻ������У�XԪ��ֻ�ܳʸ��� |
| C | Ԫ�صķǽ�����M��N | �����������M��N |
| D | Q���Ӽ���������P���Ӽ������ | Q�ķе�һ����P�ĸ� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| װ����� | �� | �� | �� | �� |
| ������װ��ͼ | 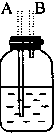 | 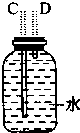 | 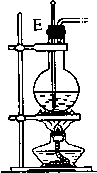 |  |

��1�����������������������ʱ������������װ�õ���ȷ����˳���ǣ����װ�õ���ţ����۽ӢٽӢڽӢܣ����Т����װ������ʱ�������������ӣ���װ������ĸ��ʾ��Ӧ��B��D��
��2��������ʱ��������¶ȹ������ɵ���ϩ�г�����SO2��Ϊ��ȥSO2��װ�â���Ӧʢ���Լ�������������Һ��
��3��װ�â������Ƭ�������Ƿ�ֹ���У�ŨH2SO4����������ˮ���ʹ�������װ���з�������Ҫ��Ӧ�Ļ�ѧ����ʽ��CH3CH2OH$��_{170��}^{Ũ����}$CH2=CH2��+H2O��
��4��ʵ�鿪ʼʱ����Ӧ���װ�õ������ԣ�ʵ�����ʱ��Ӧ�Ȳ�ȥA������װ������ĸ��ʾ���IJ������ܣ���Ϩ��۴��ľƾ��ƣ�
��5��ʵ�����ʱ��װ�â���ˮ������ڱ����Ϊ0.112L����ʵ�������������Ҵ�0.005mol��
| A�� | Na��Mg��Al��ԭ��������ǿ | B�� | PH3��H2S��HCl�ȶ���������ǿ | ||
| C�� | NaOH��KOH��Ca��OH��2�������μ��� | D�� | HCl��HBr��HI�Ļ�ԭ�����μ��� |
| A�� | 5mol | B�� | 1mol | C�� | 2.5mol | D�� | 2mol |
| A�� | NaHCO3�TNa++HCO3- | B�� | Cu��NH3��4SO4�TCu2++4NH3+SO42- | ||
| C�� | NH3•H2O?NH4++OH- | D�� | KAl��SO4��2�TK++Al3++2SO42- |