��Ŀ����
����Ŀ����ѧʽΪC2H6O�Ļ�����A�����������ʣ�A��Na�D��������������
A��CH3COOH![]() ����ζ�IJ���
����ζ�IJ���
��1������������Ϣ���Ըû�������������ж�����____����
A��һ��������OH B��һ��������COOH
C��AΪ�Ҵ� D��AΪ��ȩ
��2����A���������Ϊ75%��ˮ��Һ��������______��
��3��A���Ʒ�Ӧ�Ļ�ѧ����ʽ��_____________��
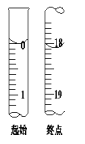
��4��������A��CH3COOH��Ӧ���ɵ�����ζ�IJ���Ľṹ��ʽΪ__________��
���𰸡�AC ҽ�������� 2Na��2CH3CH2OH�D��2CH3CH2ONa��H2�� CH3COOCH2CH3
��������
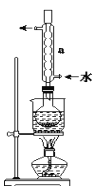
����A�Ļ�ѧʽ��A���Ʒ�Ӧ�������ݿ���֪A��һ��������OH����ѧʽΪC2H6O����Aһ��Ϊ�Ҵ���
��1�����Ʒ�Ӧ������������Ϊ������ѡAC��
��2��75%���Ҵ���Һ������ҽ����������
��3�����Ʒ�Ӧ����������2Na��2CH3CH2OH�D��2CH3CH2ONa��H2����
��4����������������������������

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ