��Ŀ����
10�����������һ�ָ�Ч���ˮ����������ҵ�ϳ�����NaClO��������������Ӧԭ��Ϊ���ټ��������£�����NaClO����Fe��NO3��3�Ƶ�Na2FeO4��
3NaClO+2Fe��NO3��3+10NaOH�T2Na2FeO4��+3NaCl+6NaNO3+5H2O
��Na2FeO4��KOH��Ӧ����K2FeO4��Na2FeO4+2KOH=K2FeO4+2NaOH
��Ҫ�������������£�
��1��д����Ӧ�ٵ����ӷ���ʽCl2+2OH-=Cl-+ClO-+H2O
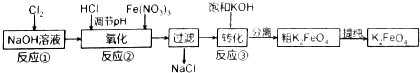
��2������ͼ�С�ת��������Ӧ�ۣ�����ij�����½��еģ�˵�����¶���Ksp��K2FeO4����Ksp��Na2FeO4���������������=������
��3����Ӧ���¶ȡ�ԭ�ϵ�Ũ�Ⱥ���ȶԸ�����صIJ��ʶ���Ӱ�죮
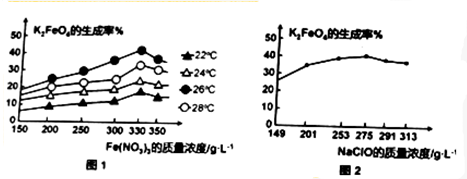
ͼ1Ϊ��ͬ���¶��£�Fe��NO3��3��ͬ����Ũ�ȶ�K2FeO4�����ʵ�Ӱ�죻
ͼ2Ϊһ���¶��£�Fe��NO3��3����Ũ�����ʱ��NaClOŨ�ȶ�K2FeO4�����ʵ�Ӱ�죮
�ٹ�ҵ����������¶�Ϊ26�棬��ʱFe��NO3��3��NaClO������Һ�������Ũ��֮��Ϊ6��5��
����Fe��NO3��3����������ڼ��Խ�����K2FeO4��Fe3+����������ԭ��Ӧ����K3FeO4���˷�Ӧ�����ӷ���ʽ��2FeO42-+Fe3++8OH-=3FeO43-+4H2O��
���� ��1���ɹ������̿�֪����Ӧ��Ϊ�������������Ʒ�Ӧ�����Ȼ��ơ�����������ˮ��
��2��������ͼ��֪����ת��������Ӧ�ۣ���Na2FeO4��K2FeO4����ת������ij�����½��еģ�˵�����¶���K2FeO4���ܽ��С��Na2FeO4��
��3������ͼ1��֪��Fe��NO3��3Ũ��һ�����¶���26��ʱ��K2FeO4����������ߣ�Fe��NO3��3Ũ����375g/Lʱ��K2FeO4����������ߣ���ͼ2��֪��NaClO��255g/Lʱ��K2FeO4����������ߣ��ݴ˼���Fe��NO3��3��NaClO������Һ�������Ũ��֮�ȣ�
������Ϣ��֪�����Խ�����K2FeO4��Fe3+����������ԭ��Ӧ����K3FeO4������Ԫ���غ㻹Ӧ����H2O����Ӧ��FeO42-��FeO43-����Ԫ�ػ��ϼ���+6�۽���Ϊ+5�ۣ��ܹ�����1�ۣ�Fe3+��FeO43-����Ԫ�ػ��ϼ���+3������Ϊ+5�ۣ��ܹ�����2�ۣ����ϼ�������С������Ϊ2������FeO42-ϵ��Ϊ2��Fe3+ϵ��Ϊ1��������Ԫ���غ�ȷ��FeO43-ϵ�������ݵ���غ�ȷ��OH-ϵ����������Ԫ���غ�ȷ��H2Oϵ���������Ԫ���غ㣮
��� �⣺��1����Ӧ��Ϊ�������������Ʒ�Ӧ�����Ȼ��ơ�����������ˮ����Ӧ���ӷ���ʽΪCl2+2OH-=Cl-+ClO-+H2O��
�ʴ�Ϊ��Cl2+2OH-=Cl-+ClO-+H2O��
��2��������ͼ��֪����ת��������Ӧ�ۣ���Na2FeO4��K2FeO4����ת������ij�����½��еģ�˵�����¶���K2FeO4���ܽ��С��Na2FeO4������Ksp��K2FeO4����Ksp��Na2FeO4�����ʴ�Ϊ������
��3������ͼ1��֪��Fe��NO3��3Ũ��һ�����¶���26��ʱ��K2FeO4����������ߣ��ʹ�ҵ����������¶�Ϊ26�森
��ͼ1��֪��Fe��NO3��3Ũ����330g/Lʱ��K2FeO4����������ߣ���ͼ2��֪��NaClO��2575g/Lʱ��K2FeO4����������ߣ�����Fe��NO3��3��NaClO������Һ�������Ũ��֮��Ϊ330g/L��275g/L=6��5���ʴ�Ϊ��26��6��5��
������Ϣ��֪�����Խ�����K2FeO4��Fe3+����������ԭ��Ӧ����K3FeO4������Ԫ���غ㻹Ӧ����H2O����Ӧ��FeO42-��FeO43-����Ԫ�ػ��ϼ���+6�۽���Ϊ+5�ۣ��ܹ�����1�ۣ�Fe3+��FeO43-����Ԫ�ػ��ϼ���+3������Ϊ+5�ۣ��ܹ�����2�ۣ����ϼ�������С������Ϊ2������FeO42-ϵ��Ϊ2��Fe3+ϵ��Ϊ1��������Ԫ���غ�ȷ��FeO43-ϵ��Ϊ3�����ݵ���غ�ȷ��OH-ϵ��8��������Ԫ���غ�ȷ��H2Oϵ��Ϊ4����Ӧ���ӷ���ʽΪ2FeO42-+Fe3++8OH-=3FeO43-+4H2O��
�ʴ�Ϊ��2FeO42-+Fe3++8OH-=3FeO43-+4H2O��
���� ���⿼��ѧ���Թ������̵����⡢�Ķ���Ŀ��ȡ��Ϣ���������ʵķ����ᴿ�Ȼ���������������ԭ��Ӧ���ܶȻ��ȣ��Ѷ��еȣ���Ҫѧ��������ʵ�Ļ���֪ʶ���������֪ʶ����Ŀ��Ϣ��������������

 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�| A�� | ����C���� | B�� | �������������Сһ�� | ||
| C�� | ����������䣬����H2O��g�� | D�� | ����ѹǿ���䣬����N2 |
 ���������з����˷ܼ�����ʧ��ƽ��Ҳ�ܻ����������£�ij���˷ܼ��Ľṹ��ʽ��ͼ��ʾ���йظ����ʵ�˵������ȷ���ǣ�������
���������з����˷ܼ�����ʧ��ƽ��Ҳ�ܻ����������£�ij���˷ܼ��Ľṹ��ʽ��ͼ��ʾ���йظ����ʵ�˵������ȷ���ǣ�������| A�� | ��������FeCl3��Һ����ɫ | |
| B�� | ��������KMnO4��Һ����ɫ��ȥ����֤����ṹ�д���̼̼˫�� | |
| C�� | 1mol�����ʷֱ���Ũ��ˮ��H2��Ӧʱ�������Br2��H2�ֱ�Ϊ4mol��7mol | |
| D�� | �÷����е�����̼ԭ�Ӳ����ܹ�ƽ�� |
| A�� | ��ع���ʱ�������������ص��������� | |
| B�� | ���缫������״���������������ĽӴ���� | |
| C�� | ������ӦΪ��O2+H2O+2e-�T2OH- | |
| D�� | �õ��ͨ��ֻ��Ҫ��������Ϳɼ���ʹ�� |
| A�� | ��CO��H2�ϳ�CH3CH2OHO�ķ�Ӧ�У�ԭ��������Ϊ100% | |
| B�� | �������м���Ũ���ᣬ���DZ��ͬʱ�����̼�����ζ�����壬˵��Ũ���������ˮ�Ժ�ǿ������ | |
| C�� | ���������Ư�۶���ʹƷ����Һ��ɫ������Ư��������ͬ | |
| D�� | ͼ�����з�Ӧ��Ϊ������ԭ��Ӧ |
�ټ���Ҵ����Ƿ���ˮ�ɼ�����������ˮ����ͭ���������ˮ
�ڳ�ȥ�Ҵ�����ˮ�ɼ�������ƣ�ʹ����ȫ��Ӧ
�۳�ȥ������������ϩ�ɹ����Ը��������Һϴ��
�ܻ����ˮ�Ҵ��ķ���ͨ������������ʯ����ˮ��Ȼ���ټ�������
| A�� | �٢� | B�� | �ڢ� | C�� | �٢� | D�� | �ۢ� |
| A�� | ��ϩ����Ϊƽ��ṹ���������Ϊ�ռ�����ṹ | |
| B�� | ��ϩ����������ж����м��Լ��ͷǼ��Լ� | |
| C�� | ��ϩ�ܷ����ӳɷ�Ӧ�������鲻�� | |
| D�� | ��ϩ�������C�TC˫�����������������C-C�������ܵ����� |
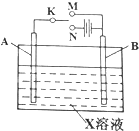 ������ͼװ�ã����Խ�������绯ѧʵ�飮
������ͼװ�ã����Խ�������绯ѧʵ�飮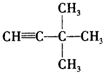 ��3��3-����-1-��Ȳ��
��3��3-����-1-��Ȳ��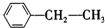 ���ұ���
���ұ���